Cyanidin and its glycosides belong to the anthocyanins, a class of water-soluble plant compounds responsible for the brilliant color (red, orange, blue) of fruits and flowers. Anthocyanins have a variety of health benefits due to their peculiar chemical structure. Cyanidin is commonly used as a food colorant and in supplements and nutraceuticals.
What is Cyanidin?
Cyanidins occur mainly as glycosides of their respective aglycone anthocyanidin-chromophores. Glucose, galactose, arabinose, rutinose, rhamnose, and xylose are the most common sugars bonded to cyanidins as mono-, di-, or trisaccharide forms. The sugar moieties mainly attach to the 3-position on the C-ring or the 5, 7-position on the A-ring. Due to cyanidins’ electron deficiency, they are reactive towards reactive oxygen species (ROS).
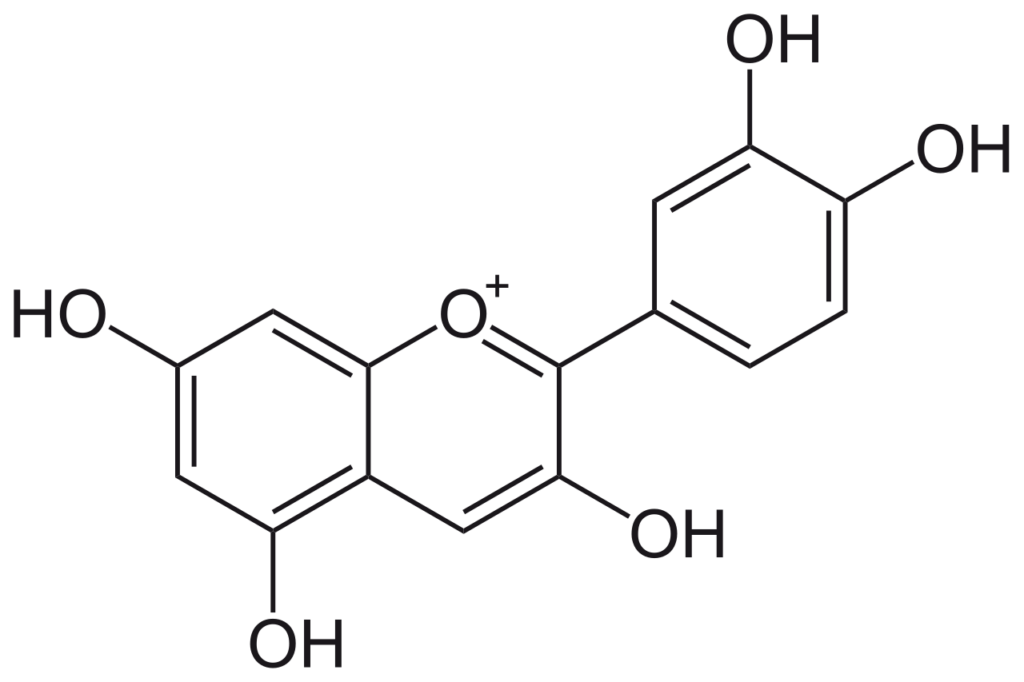
Source: Wikipedia
Sources of Cyanidin
Cyanidins are considered the widest-spread anthocyanin in the plant kingdom. They are a common component of plant-based diets in beans, fruits, vegetables, red wine, and other plant-based foods.
Distributions of cyanidin pigment in various fruits and vegetables are shown in the following table.
| Fruit/Vegetable | Cyanidin (mg/100g) |
| Chokeberry, raw | 344.07 |
| Aronia/ chokeberry juice concentrate | 231.61 |
| Black currant, raw | 62.46 |
| Black currant juice | 29.76 |
| Blueberries, raw | 8.46 |
| Carrot, black/ purple | 13.68-17.58 |
| Cherries, sweet raw | 30.21 |
| Cabbage, red | 209.83 |
| Elderberries, raw | 485.26 |
| Elderberry juice concentrate | 411.4 |
| Grapes, red raw | 1.16 |
| Grape juice, red | 0.4 |
| Potato, purple | 0.02-0.14 |
| Radish, red | 0 |
Source: USDA
Use of Cyanidin in Food and Nutrition
Cyanidin is commonly used as a coloring agent in various foods, including bakery items, beverages, confectionery, snacks, desserts, dairy products, and chocolates. The resulting color is pH-dependent.
Besides being used as a food additive for color, cyanidin also has various health benefits. Due to these benefits, it’s also used in supplements and nutraceuticals.
Properties of Cyanidin
| Physical Form | Powder |
| Color | Red to Blue (pH dependent) |
| Storage Temperature | 2-8℃ |
| Molecular Weight | 287.244 |
| Log P | 2.90890 |
| Melting Point | >300°C |
Cyanidin Formulation Considerations
Variants
Cyanidins are present in their glycosidic forms as mono-, di-, or trisaccharides. Cyanidin-3-glucoside is the most abundantly found glucoside of cyanidin. The types of cyanidin glycosides & other derivatives of cyanidin naturally present in various food sources are listed in the following table.
| Source | Cyanidin/Cyanidin Glycoside |
| Elderberry extract | Cyanidin-3-sambubioside, Cyanidin-3-glucoside, Cyanidin-3-rutinoside |
| Blackcurrant juice | Cyanidin-3-glucoside, Cyanidin-3-rutinoside |
| Boysenberry concentrate | Cyanidin-3-glucoside, Cyanidin-3-sophoroside |
| Purple sweet potato beverage | Acylated Cyanidin |
| Purple carrots | Acylated Cyglycosides |
| Blackberries | Cyanidin-3-glucoside |
| Hibiscus sabdariffa extract | Cyanidin-3-sambubioside |
| Freeze-dried black raspberries | Cyanidin-3-rutinoside, Cyanidin-3-xylosylrutinoside |
| Chokeberry extract | Cyanidin-3-galactoside, Cyanidin-3-arabinopyranoside |
Source: AISS
Stability
The stability of cyanidin can be affected by factors such as pH, temperature, concentration, light, solvents, the presence of oxygen, enzymes, other flavonoids, proteins, and metallic ions.
In aqueous solutions, anthocyanins undergo structural rearrangements in response to changes in pH and convert to flavylium cation, quinoidal base, carbinol, and chalcone forms. Anthocyanins are most stable in acidic solutions (pH 1–3), where they exist primarily as flavylium cations. At pH above 4, anthocyanins adopt the forms of carbinol and chalcone. Chalcone can then undergo chemical degradations to produce phenolic acids.
Color
The distribution of the different anthocyanins and the resulting hue, strength, and stability of color varies across various fruits and vegetables. The color of the pigments also depends on the solution’s pH. Red is typical at acidic (low) pH, while purple or violet colors usually occur at neutral pH. Blues are most common at moderately basic pH, turning to green or yellow at very high (basic) pH levels.
Biological Activity
The natural electron deficiency of anthocyanins makes these compounds particularly reactive and is responsible for their antioxidant activity. However, this property also makes them very sensitive to pH and temperature changes. The following table describes formulation considerations based on the biological activity of cyanidin.
| Antioxidant Activity | – The antioxidative mechanism of cyanidin-3-glucoside is different from that of alpha-tocopherol. Based on study results, it is hypothesized that C3G produces another radical scavenger. – Cyanidin is a potent antioxidant. The antioxidant activity of cyanidin-3-glucoside is ~3.5 times that of Trolox (Vitamin E analog). – Cyanidin-3-glucosylrutinoside and cyanidin-3-rutinoside have antioxidant properties equivalent to TBHQ, BHT & BHA. – Cyanidin glucosides inhibit lipid peroxidation of the liposome system. In a study, the inhibition varied from 39-75%, where aglycones had higher activity than glycosides. – Cyanidin-3-glucoside was reported to inhibit the Cu2+ mediated human low-density lipoprotein (LDL) oxidation higher than resveratrol and ascorbic acid and was independent of pH variations from 4 to 7.4. – In a study, authors showed that feeding rats with cyanidin-3-glucoside lowered the serum thiobarbituric acid-reactive substance (TBARS) concentration and increased the oxidation resistance of the serum to lipid peroxidation provoked by 2,2-azobis(2-amidinopropane)hydrochloride or Cu2+. |
| Anti-mutagenic Properties | – In a study, 3-(6,6′-caffeylferuly sophoroside)-5-glucoside of cyanidin showed a powerful antimutagenic capacity which was not influenced by deacylation. – Another study showed that cyanidin-3-glucoside demonstrated antimutagenic activity against 2-aminoanthracene-induced mutagenicity (23-90%) in tested microorganisms. |
| Anti-carcinogenic Activity | – Purple corn color, a natural food colorant mainly composed of cyanidin-3-glucoside, has been shown to suppress the incidence and multiplicity of colorectal adenomas and carcinomas induced by carcinogens. – Proanthocyanidin fractions of blueberry, cranberry, and lingonberry showed active inhibition of the ornithine decarboxylase activity, and ornithine decarboxylase is a key enzyme in polyamine biosynthesis. – In a study, cyanidin strongly inhibited the epidermal growth factor receptor by shutting off the downstream signaling cascades in human vulva carcinoma cells. |
| Anti-inflammatory Activity | – Cyanidin-3-glucoside can reduce the level of inflammatory factors such as tumor necrosis factor‐a (TNF‐a), interleukin (IL)‐1β, IL‐6, and IL‐8, stimulated by lipopolysaccharide (LPS). It also can protect macrophages from apoptosis. – In a study, cyanidin exhibited inhibitory activity on prostaglandin H endoperoxide synthase-1 & 2, showing anti-inflammatory properties. – Cyanidin-DNA copigmentation complex has been shown to protect DNA from oxidative damage by free hydroxyl radicals. |
Absorption & Metabolism
- Absorption: Cyanidin-glycosides are incorporated from the digestive tract into the bloodstream in their intact glycosylated forms. A study reported the presence of cyanidin-glucosides in the bloodstream after the oral administration of cyanidin-3-glucoside, cyanidin-3,5-diglucoside, and cyanidin 3-rutinoside.
- Metabolism: Studies have reported no detectable amounts of cyanidin in plasma or the liver despite oral administrations of cyanidin-3-glucoside and cyanidin-3,5-diglucoside. It is hypothesized that no hydrolysis of the glycosidic bond occurs in the gastrointestinal tract. However, hepatic methylation at the 3-hydroxyl moiety position of cyanidin-3-glucoside has been reported in a study. Cyanidin in its aglycone form was reportedly found in the jejunum, which supports the possibility of its partial hydrolysis by enzyme ꞵ-glycosidase in the intestine. Protocatechuic acid was the predominant degradation product of cyanidin in a culture cell study. The instability of cyanidin in plasma and its degradation to protocatechuic acid can be attributed to its absence in plasma on consumption.
- Excretion: In a study, cyanidin-3-glucoside and cyanidin-3,5-diglucoside were excreted in their intact glycosylated forms in the urine of humans. The elimination of plasma total anthocyanins appeared to follow first-order kinetics since the urinary excretion rate had a maximum after three to four hours and decreased after that. The primary metabolites of anthocyanins recovered in urine are glucuronidated and/or methylated conjugates.
Bioavailability
In animal studies, the systemic bioavailability of anthocyanins is only 0.26–1.8%. The percentage of intact anthocyanins excreted in urine is estimated to be less than 0.1% in humans. Between 30% and 44% of consumed cyanidin-3-glucoside has been found as protocatechuic acid in human plasma.
Specific dietary components are reported to influence the bioavailability of cyanidins. Coadministration with milk and sucrose is reported to delay absorption and reduce availability. Carbohydrate-rich diets are reported to delay absorption by prolonging the transit of anthocyanins through the gastrointestinal tract.
The presence of other flavonoids may interfere with the absorption of anthocyanins. A study found cyanidin-3-glucoside to be more stable when present in blackberry extract (0.69% decomposed) than in its pure form (2.3% decomposed).
Safety and Regulatory Considerations
| FDA Information | – Cyanidins are included in the National List as agricultural products derived from fruit, vegetable, root, flower, or algal sources used to add color to various food products. They are allowed as ingredients in or on processed products. – When used as a fruit or vegetable juice component for adding color, cyanidin falls under the list of color additives exempt from certification as per 21 CFR 73. |
| EU Information | According to the EFSA, anthocyanins (E 163) are authorized as food additives in the EU. JECFA has established an ADI of 2.5 mg/kg bw/day for anthocyanins from grape skin, while the SCF has not derived an ADI for anthocyanins. |
Health Effects of Cyanidin
- Cardioprotective: Cyanidin is reported to regulate the endothelial nitric oxide synthase. For this reason, it exerts a vasoprotective effect against peroxynitrite-induced endothelial dysfunction and vascular failure.
- Neuroprotective: In a study encompassing cyanidin-3-glucoside, a neuroprotective effect was observed concerning PC12 cells exposed to cell-damaging oxidative stress.
- Gastroprotective: Cyanidin-3-glucoside has been shown to have a strong gastroprotective effect on acute gastric hemorrhagic lesions in rats.
- Anti-Obesity: In a study, dietary purple corn color consisting mainly of cyanidin-3-glucoside significantly suppressed a high-fat diet-induced increase in body weight and white and brown adipose tissue weights in rats. In another study, cyanidin demonstrated an up-regulation of hormone-sensitive lipase and the enhancement of lipolytic activity.
- Anti-Diabetes: Dietary purple corn color consisting mainly of cyanidin-3-glucoside has been shown to prevent hyperglycemia and hyperinsulinemia. In a study, cyanidin-3-glucoside stimulated in vitro insulin secretion from rodent pancreatic beta-cells.
Safety & Toxicity of Cyanidin
Toxicological studies are limited and have been carried out with mixtures extracted from various fruits. The available data indicate that such extracts are of a very low order of toxicity.
Identification Numbers
| IUPAC Name | 2-(3,4-dihydroxyphenyl)chromenylium-3,5,7-triol |
| CAS Number | 13306-05-3 |
| EC Number | 230-384-7 |
| E Number (Food Additive) | E 163 |
| INS No. (Food Additive) | INS 163 |
Acceptable Limits or Maximum Usage
The acceptable daily intake of cyanidin, as per JECFA is 0-2.5 mg/kg bw.
Fun Facts About Cyanidin
- Anthocyanins, including cyanidin, may play a role in plant defense against environmental stressors such as UV radiation, pests, and diseases. They are thought to protect by absorbing harmful UV rays and deterring herbivores.
- Cyanidin levels can impact the color and ripening of fruits. For example, blueberries become more bluish-purple as they ripen due to increased cyanidin content.








