Dihydrokaempferol, also known as aromadendrin, belongs to the class of organic compounds known as flavanonols in the flavonoid family. Flavonoids are secondary plant metabolites and have been widely reported to exhibit various pharmacological activities, including anti-inflammatory, anti-oxidant, anti-tumor, and neuroprotective effects. Due to its health benefits, dihydrokaempferol is an additive in dietary supplements, nutraceuticals, and functional foods.
What is Dihydrokaempferol?
The antioxidant activity of phenolic compounds and flavonoids depends on the number of hydroxyl groups in the structure. Dihydrokaempferol is a tetrahydroxyflavanone, having hydroxy groups at the 3-, 4′-, 5- and 7-positions. Flavanonols are compounds containing a flavan-3-one moiety, with a structure characterized by a 2-phenyl-3,4-dihydro-2H-1-benzopyran bearing a hydroxyl group and a ketone at the carbon C2 and C3, respectively. Dihydrokaempferol is an extremely weak basic (essentially neutral) compound (based on its pKa).
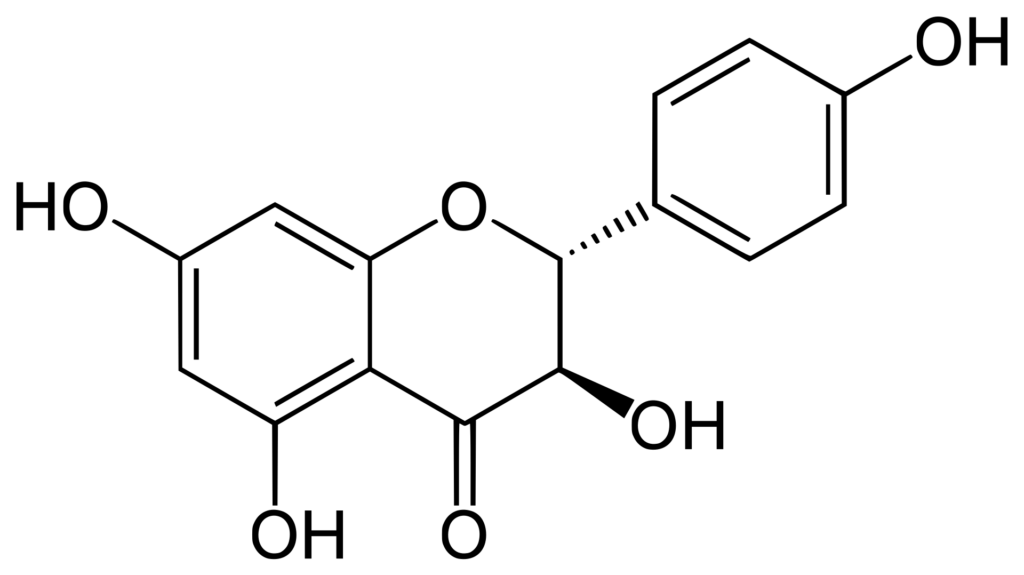
Source: Wikipedia
How is Dihydrokaempferol Produced?
Dihydrokaempferol has been detected in several plants, including anises, sorrels, tamarinds, wax apples, and sea-buckthorn berries. It has also been extracted from the Rutaceae family of fruits (Citrus paradisi), Scots pine, and Opuntia ficus-indica. The concentration of dihydrokaempferol in different parts of the plant varies. The following table lists the concentration of dihydrokaempferol observed in different parts of Manilkara zapota.
| Plant Part | Solvent Extract: Content of (+)-dihydrokaempferol (mg/g of crude extract) |
| Bark | Methanol: 33.62±0.01 Water: 27.94±0.01 |
| Flowers | Methanol: 11.30±0.01 Water: 9.12±0.02 |
| Leaves | Methanol: 8.46±0.01 Water: 5.70±0.01 |
| Roots | Methanol: 23.20±0.01 Water: 12.44±0.01 |
| Wood | Methanol: 22.96±0.01 Water: 10.10±0.01 |
Source: IJPS
Properties of Dihydrokaempferol
| Physical Form | Powder |
| Storage Temperature | 0-8°C |
| Molecular Weight | 288.25 g/mol |
| Melting Point | 247-249°C |
| Boiling Point | 638.00-639.00°C |
| LogP | 2.860 |
| Solubility (25°C) | 2.136e+004 mg/L |
Dihydrokaempferol Formulation Considerations
Dihydrokaempferol vs. Kaempferol
Flavones and flavonols occur as aglycones in foods. Flavonones and flavonols are characterized by a saturated C2 single bond, a C3 bond, and an oxygen atom (carbonyl group) in the 4-position. For this reason, flavonones may be referred to as dihydroflavones. Flavononols differ from flavonones because they have a hydroxyl group in the 3-position and are often referred to as 3-hydroxyflavonones or dihydroflavonols.
Dihydrokaempferol acts as a precursor for kaempferol. In Cynodon dactylon, naringenin is converted into kaempferol via dihydrokaempferol by flavanone 3β-hydroxylase (F3H) and flavonol synthase (FLS). Dihydrokaempferol7-glucoside has been shown to undergo oxidation to kaempferol-7-glucoside in boiling water.
Biological Activity
The following table describes formulation considerations based on the biological activity of dihydrokaempferol.
| Antioxidant | – In a study, extracts of different parts of the M. zapota plant high in dihydrokaempferol (~33 mg/g extract) showed potent antioxidant activity ~10 times that of the commercial antioxidant Trolox. – (2S,3S)-dihyrokaempferol 3-O-b-D-glucoside and its stereoisomer extracted from acai have been shown to exhibit strong anti-oxidant activity. |
| Anti-Inflammatory | – In a study, anti-inflammatory effects were reported for (2S,3S)-dihyrokaempferol 3-O-b-D-glucoside and its stereoisomer, screened by secreted an embryonic alkaline phosphatase (SEAP) reporter assay to measure NF-jB activation. Inhibition of SEAP secretion induced by oxidized LDL indicates potential atheroprotective effects. – Another study revealed that aromadendrin exhibited an anti-inflammatory mechanism in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. |
| Tyrosinase inhibitor | – In a study, extracts of different parts of the M. zapota plant high in dihydrokaempferol (~33 mg/g extract) showed potent antioxidant activity ~10 times that of the commercial Trolox. – (2S,3S)-dihyrokaempferol 3-O-b-D-glucoside and its stereoisomer extracted from acai have been shown to exhibit strong anti-oxidant activity. |
| Antiviral Activity | – Dihydrokaempferol could inhibit the activity of SARS-CoV-2 main protease and HCoV-229E main protease, an essential enzyme required to multiply these two viruses in the host cells. The activity is attributed to forming hydrogen bonds with different amino acids in the binding pocket. – In a study, dihydrokaempferol-3-glucoside showed antiviral activity against SARS CoV-2 virus by inhibiting the main protease in DNA replication. |
| Anti-Cancer Activity | Crude extracts of bark and root of M. zapota, high in dihydrokaempferol, show strong monophenolase & diphenolase inhibitory activity. |
| Neuroprotective | – In a study, ethanol extracts of Bidens pilosa with dihydrokaempferol exhibited cytotoxic activity on HepG2 (cancerous) and Vero (non-cancerous) cell lines. In contrast, the water extracts promoted cell proliferation at selected concentrations. – Dihydrokaempferol has exhibited inhibitory effects on the proliferation of synoviocytes. It also effectively promotes apoptosis. |
| Cardioprotective | Dihydrokaempferol has been shown to have an inhibitory effect on cardiac hypertrophy by down-regulating NFAT and MAPKs pathways. |
| Immunosuppressive Agent | In a study, a suppressive effect of aromadendrin was reported on T cell activation by Ca2+ influx regulation through NFAT activity suppression of the activated T cells. |
Safety & Regulatory Considerations
| FDA Information | Dihydrokaempferol is listed in the Global Substance Registration System – GSRS & DAILYMED database. It was first approved for use in 2014. |
| EU Information | Dihydrokaempferol is listed as an intermediate or product resulting from metabolism in the Chemical Entities of Biological Interest (ChEBI) database. |
Identification Numbers
| IUPAC Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one |
| CAS Number | 480-20-6 |
| EC Number | 687-473-2 |
Fun Facts About Dihydrokaempferol
- Flavonoids often work together in plants, potentially enhancing each other’s effects. This phenomenon, known as the “flavonoid synergy,” suggests combining various flavonoids in foods may provide more significant health benefits than isolated compounds.
- Due to their antioxidant and anti-inflammatory properties, some flavonoids are used in cosmetic and skincare products. While dihydrokaempferol might not be as commonly used as other flavonoids, its potential benefits could also extend to these applications.



