The amino sugar N-Acetylglucosamine (GlcNAc or NAG) is the acetylated derivative of glucosamine which is one of the building blocks of joint tissue and other connective tissues in the body. NAG is also a component of the cartilage matrix and synovial fluid. It is well known for its essential structural roles on the cell surface. N-acetylglucosamine is also a key component of bacterial cell wall peptidoglycan, fungal cell wall chitin, and the extracellular matrix of animal cells. NAG is recognized as the natural precursor of hyaluronic acid and is used in nutraceutical supplements for chondroprotection.
What is N-Acetylglucosamine?
N-Acetylglucosamine is an amino monosaccharide derivative of glucose. Chemically, NAG is an amino water-soluble monosaccharide, resulting from the link of glucosamine (GA) and acetic acid and polymerizes linearly with (1,4)-β-linkages.
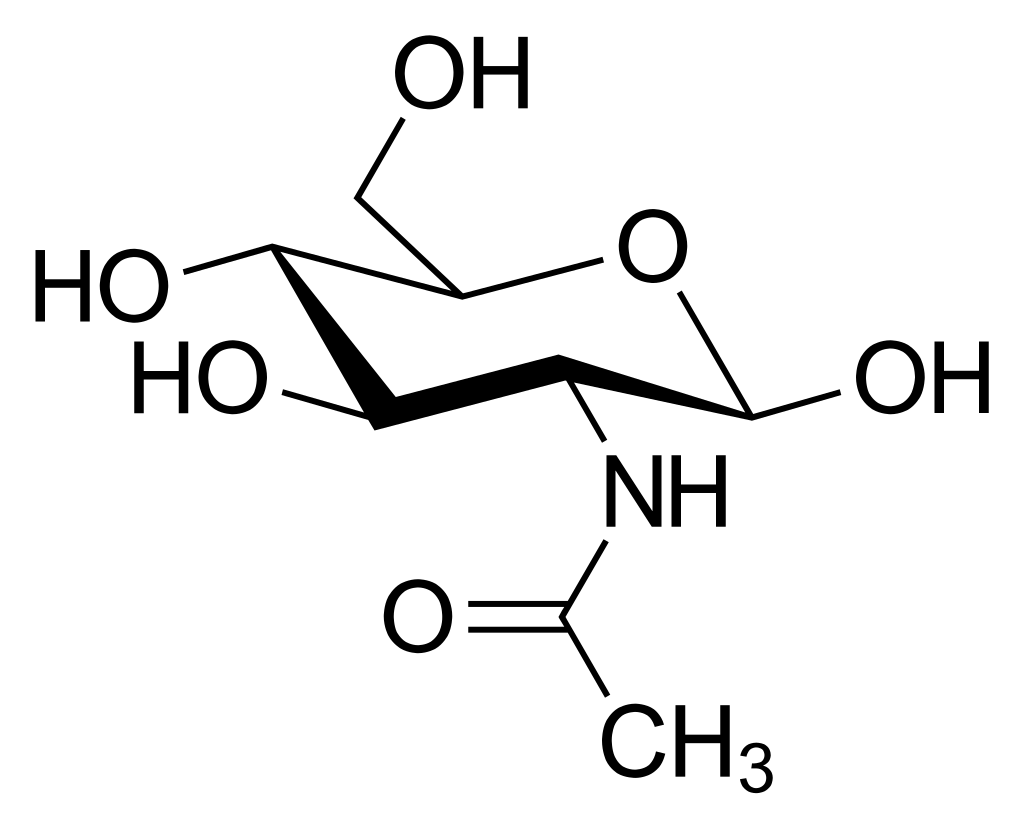
Source: Wikipedia
How is N-Acetylglucosamine Produced?
N-Acetylglucosamine is produced via chemical, enzymatic, and biotransformation methods using chitin as the substrate. GlcNAc is also obtained using glucose as a substrate from genetically modified microorganisms.
- Natural Occurrences: N-Acetylglucosamine is the monomeric unit of the polymer chitin, the second most abundant carbohydrate after cellulose. It is also a constituent of heterogeneous polysaccharides, such as murein and hyaluronic acid. NAG and sulfated NAG can be isolated from heparin or keratin sulfate. It seldom exists in free form, except in human milk (600–1500 mg/mL).
- Chitin Hydrolysis: Using chitin as feedstock, NAG can be prepared through a process that is based on chitin hydrolysis.
| Chemical Method | – Hydrolyzed using strong acids such as HCl – Suitable reaction conditions include 15–36% HCl and ~40–80°C (enough to degrade chitin but preserve GlcNAc) – Economically advantageous but produces a low yield (below 65%) and acidic waste. – Not considered a natural material due to its chemical modification. |
| Enzymatic Method | – Chitinolytic enzymes can contain endochitinases, exochitinases, chitobiosidases, and N-Acetylglucosaminidases – High yield (up to 77%) & purity (~100%) but low productivity – High cost for specific enzymes to be isolated – Low susceptibility of natural α-chitin due to its high crystallinity is a drawback |
| Biotransformation | – Chitin degradation using microbesHigh yield (up to 75%), low purity, high productivity – Genetic modification of microorganisms can be done. |
Use of N-Acetylglucosamine in Nutrition Products
N-Acetylglucosamine serves as a dietary supplement in nutrition products, including capsules, liquid, and powder supplements.
| Capsules | – Easiest dosage form, with stable and extensive shelf life – Readily available in doses from 200 mg to 1500 mg per tablet |
| Liquid | – Has a slightly sweetish taste and can be added to liquids that have a sweet taste or are not noticeably affected by sweetening – Has reasonable solubility in water (~50 mg/ml) and thus can be used in liquid formsIssues with shelf life (Since NAG is an amino sugar, it is subject to fermentation by microbes.) |
| Powder | – Easy to mix powder – Can be mixed with foods in required amounts |
Properties of N-Acetylglucosamine
| Physical Form | Powder |
| Color | White |
| Taste | Slightly sweet |
| Shelf Life | 2 years |
| Storage Temperature & Conditions | Dry and well-ventilated place at -20°C for longer shelf life |
| Molecular Weight | 221.21 |
| Appearance | White powder |
| pKa | 11.6 (Strongest Acidic)-0.78 (Strongest Basic) |
| Melting Point | 221°C |
| Solubility | 25% in water (50 mg/mL) |
Typical Formulations
Probiotic Formulation
Here is an example of a probiotic formulation table with N-Acetylglucosamine along with the % weight of ingredients:
| Ingredient | % Composition |
| Probiotic mixture (Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus salivarius, Bifidobacterium infantis, and Bifidobacterium longum) | 30-35% |
| Fructooligosaccharides | 20-30% |
| L-glutamine | 30-35% |
| N-Acetylglucosamine | 10-15% |
Source: Google Patents
Formulation Considerations
Variants
N-Acetylglucosamine is a monomeric unit of the polymer chitin. Naturally, chitin is found in three crystalline polymorphic forms:
- α-chitin has antiparallel chains.
- β-chitin has parallel chains.
- γ-chitin has a mixture of parallel and anti-parallel chains.
The rarer β-chitin is found in squid pens and is commercially more expensive as it cannot be obtained from solution or in vitro biosynthesis.
Comparison with Other Forms of Glucosamine
Glucosamine sulfate, glucosamine hydrochloride, and N-Acetylglucosamine are different forms of glucosamine. NAG differs from glucosamine sulfate and glucosamine hydrochloride — instead of a sulfur or chloride molecule, NAG has a larger, more complex molecule attached to it. Like other forms of glucosamine, NAG is taken to help support joint tissue, but it is also used as an anti-aging ingredient for skin and may provide some support for digestive health. Also, unlike glucosamine, NAG does not promote HA synthesis in synovial cells and chondrocytes. Conversely, it upregulates the hyaluronan synthase-2 in human articular chondrocytes.
Biological Activity
Antioxidant Activity: The antioxidant ability of N-Acetylglucosamine is due to its superoxide/hydroxyl-radical scavenging capabilities. NAG has been shown to have a cytoprotective property against serum glucose deprivation-induced cell death via antioxidant activities. The upregulation of antioxidant enzymes (catalase, GSH S-transferase, peroxiredoxin, glutathione peroxidase, paraoxonase) also contributes to alleviating the pro-oxidant activity.
Anti-Inflammatory Activity: An exaggerated immune response triggered by neutrophils can lead to damage to nearby cells and the extracellular matrix. N-Acetylglucosamine helps to suppress inflammation in the body, which could assist in managing autoimmune and inflammatory diseases. The anti-inflammatory effect of NAG is owed to multiple factors, including suppression of neutrophil function (superoxide generation, phagocytosis, and neutrophil chemotaxis). N-Acetylglucosamine also inhibits IL-1beta- and TNF-alpha-induced nitric oxide production in human articular chondrocytes, thus inhibiting inflammation.
Cellular Functions in Humans: N-Acetylglucosamine molecules with various functional group substitutions are directly involved in cellular interactions. NAG appears to be the basic element of the H antigen in ABO blood grouping. NAG has also been identified as a part of ligands that capture selectins for adhesion and contribute significantly to metastasis. Glycoproteins that contain N-Acetylglucosamine can be found in the mucous membranes of the digestive tract. Their saccharide modification may promote pathogen binding. O-linked NAG typically modifies nuclear pore proteins involved in nuclear pore assembly and function. NAG is frequently observed in glycoproteins, such as tissue plasminogen activators, as well as many mammalian growth factors and hormones.
Cellular Functions in Plants: In plants, N-Acetylglucosamine has been found in bromelain, ricin agglutinin, and abrus agglutinin. It functions as a microbial signaling molecule to shape the community structure and metabolism of the rhizosphere microbiome, thereby regulating plant growth and development. It also serves as plant disease prevention during plant–microbe interactions.
Absorption & Excretion
N-Acetylglucosamine is absorbed in the intestine by a simple diffusion process without deacetylation of the molecule. Unfortunately, the half-life of glucosamine in the blood is relatively short. In a study, 54% of the administered dosage was excreted into the urine in one day.
For this reason, a sustained-release form of the compound would be highly desirable.
Combination Supplements
N-Acetylglucosamine is often found in combination formulas for joint health and ingredients that work together to nourish joint tissues and promote mobility. NAG and chondroitin can be synergistically utilized to support joints’ cartilage structure and cushioning function. Additionally, methylsulfonylmethane (MSM) — a highly bioavailable form of sulfur — is often added to promote healthy connective tissues like tendons, ligaments, and other critical joint tissues. It also works as an antioxidant to help protect tissue cells.
Bioavailability
Preparations containing N-Acetylglucosamine are delivered by parenteral, oral, transmucosal, and topical administration. Approximately 90% of orally administered NAG gets absorbed from the small intestine. The increase in serum glucosamine level indicates in-vivo the conversion of NAG to glucosamine. NAG shows a peak of adsorption four hours after the administration, with residual activity maintained up to 168 hours post-administration.
Safety & Regulatory Considerations
| FDA Information | The FDA approves using N-Acetylglucosamine for topical use in compound drug products. However, despite promising clinical and preclinical research, NAG supplements have not been approved by the FDA for medical use. |
| EU Information | Chitin complexes composed of N-Acetylglucosamine are authorized by the EU to be used under the novel food category. |
Health Benefits of N-Acetylglucosamine
Joint Health
Glucosamine and N-acetylglucosamine build complex elastic molecules in the cartilage that lubricate the joints and enable smooth movement. This improves collagen production, may enhance cartilage recovery, and repair the joints. At 500 mg and 1,000 mg/day doses, NAG has been observed to exhibit a chondroprotective effect in healthy individuals by improving cartilage metabolism. The administration of NAG has been shown to enhance type II collagen synthesis and help with knee osteoarthritis.
Gut Health
N-acetylglucosamine can promote the growth and development of the intestinal tract and improve the digestion and absorption capacity of the intestine by regulating the activity of intestinal stem cells. NAG administration can alleviate Inflammatory Bowel Disease (IBD)-related inflammation by increasing glycosaminoglycan synthesis, resulting in more glycosaminoglycan attachments to the protective mucin layer. NAG is one of the metabolites that can also be specifically sensed by gut pathogens as signals to induce or repress virulence genes. It has been reported to reduce the biofilm formation of E. coli strains.
Multiple Sclerosis Prevention & Management
N-Acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. NAG can act as critical regulators of primary myelination and myelin repair. Oral administration of NAG may be neuroprotective in demyelinating diseases like multiple sclerosis.
Wound Healing & Bleeding Control
Polymers of N-Acetylglucosamine have been used for their wound-healing properties. In a study, supplementation of keratinocytes with NAG led to a dose-dependent increase of hyaluronic acid (HA). The wound-healing properties of NAG are likely due to its ability to increase HA in the skin. In another study, N-Acetylglucosamine nanofibers significantly improved wound healing.
Skin Rejuvenation
N-Acetylglucosamine helps to rejuvenate the skin by reducing wrinkles and other signs of aging, hydrating the skin, and speeding up wound healing. NAG can reduce the amount of matrix metalloproteinases in the skin, which are responsible for collagen degradation. NAG is a precursor for hyaluronic acid (HA) biosynthesis in the body. The main role of HA is to preserve the hydration and elasticity of the skin. NAG-induced changes in the skin fibroblasts’ properties may be important for preventing age-dependent changes in their structure and function.
Safety & Toxicity of N-Acetylglucosamine
N-Acetylglucosamine is safe when used in intravenous, oral, and topical formats.
Toxicity tests reveal that NAG is non-toxic. The half-life of NAG with 20g intravenous injection is 220 minutes. While NAG is safe for most people, it’s important to consider the following:
- NAG may cause minor difficulties such as nausea, heartburn, or diarrhea. It may also worsen diabetes and glaucoma as it increases insulin resistance.
- NAG may interact with blood thinners.
- NAG obtained from shellfish is not suitable for people with shellfish allergies.
Identification Numbers
| N-Acetylglucosamine IUPAC Name | N-[(2R,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
| CAS Number | 7512-17-6 |
| EC Number | 231-368-2 |
| ChEMBL ID | CHEMBL447878 |
Fun Facts About N-Acetylglucosamine
- N-Acetylglucosamine is a crucial component of chitin, a tough, insoluble substance found in the exoskeletons of arthropods (such as insects and crustaceans) and the cell walls of fungi.
- Certain insects, like beetles, release N-Acetylglucosamine as a defense mechanism against predators.