Alginate is a polysaccharide derived from brown seaweed. Due to its gel-forming properties, alginate is commonly used in the food industry to modify certain food characteristics, such as rheology (thickening), water binding capacity, stabilizing emulsion, and film formation. One of the most highly valued properties of alginate is its ability to form an ionic gel in the presence of multivalent cations, irrespective of heating requirements. The gel formed by this interaction is used to encapsulate bioactives.
What is Alginate?
The alginate polymer is made up of two monomeric units: ꞵ(1-4) linked D-mannuronic acid (M) residues and α-(1-4)-linked L-guluronic acid (G) residues. The basic structure of alginates consists of linear unbranched units of polymers made up of monomers arranged in blocks of M and G residues interspersed with regions containing alternating M-G sequences within the structure. Alginates do not have regularly repeating units and the distribution is uneven.
Commercially, alginates are available in the form of calcium, sodium, potassium, or ammonium salts. Molecular weights of alginate typically range from 60,000 to 700,000 Daltons depending on the application.
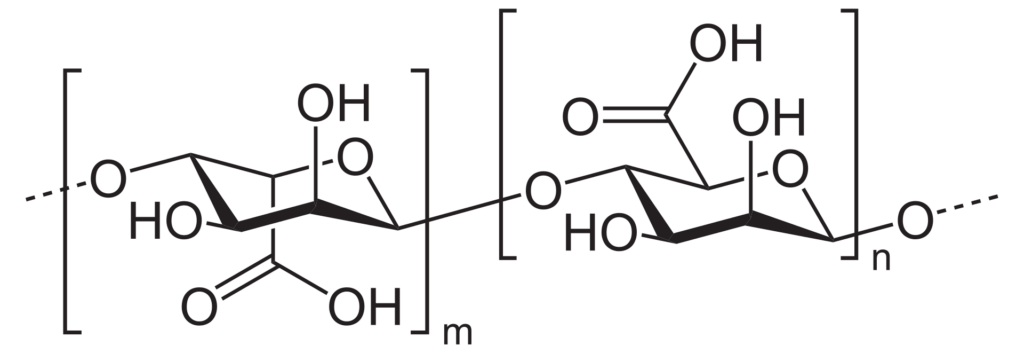
Source: Wikipedia
How is Alginate Produced?
Alginates are a part of the cell wall and inter-cellular matrix of brown seaweeds. Seaweed alginates exist as an insoluble mixed salt of all cations that are found in seawater such as calcium, sodium, and magnesium. The alginate biopolymer can represent up to 40% of the dry matter of brown seaweeds.
Alginates are produced industrially via aqueous extraction from marine seaweeds that belong to the taxonomic group of brown algae (class Phaeophyceae). Alginate-yielding seaweeds, also called “alginophytes,” are mainly harvested from wild populations, although they can also originate from the artificial cultivation of algae. Commercially used seaweeds include genera Ascophyllum, Durvillaea, Ecklonia, Laminaria, Lessonia, Macrocystis, and Saccharina.
Generally, the protocol for aqueous extraction of alginates is composed of five steps: acidification of the seaweeds, alkaline extraction with sodium carbonate (Na2CO3), solid/liquid separation, precipitation, and drying.
Applications in the Food Industry
Alginate has several uses in the food industry due to its unique properties. Here are some common uses of alginate in food:
| Function | Description |
| Thickener | Alginate is an effective thickening agent in food products and helps improve their consistency. |
| Gelling Agent | Alginate can form a gel when it interacts with calcium ions. This property is used in various food applications, such as creating gelled desserts, jellies, and gummies. |
| Encapsulant | Alginate can encapsulate flavors, nutrients, and other active ingredients. This allows for the controlled release and protection of active ingredients in food products. |
| Stabilizer | Alginate can stabilize emulsions by preventing the separation of oil and water. It can be used in salad dressings, sauces, and mayonnaise to improve their stability and prevent oil and water from separating. |
| Coating Agent | Alginate can be used as a coating for fresh produce to extend shelf life. The coating forms a protective barrier that helps to reduce moisture loss and prevent microbial growth. |
| Moisture Retention Agent | Alginate can retain moisture, which can help improve the shelf life of food products. It can also prevent moisture loss in baked goods, meat products, and processed foods. |
| Texturizer | Alginate can improve the texture of food products by providing a smooth and creamy mouthfeel. It can be used in dairy products, such as yogurt and ice cream, to enhance their texture and creaminess. |
| Clarifying Agent | Alginate can help clarify suspended particles in beverages. |
| Fat Replacer | Alginate can be effectively used to replace fat in recipes. |
Product Examples
| Type | Examples |
| Dairy | Yogurt, ice cream, cheese |
| Bakery | Bread, cake, pastries |
| Confectionery | Gummies, jellies, jams |
| Sauces & Dressings | Salad dressings, sauces, gravies |
| Meat & Seafood | Sausages, meatballs, fish products |
| Desserts & Puddings | Puddings, fruit jellies |
| Beverages | Milk-based beverages, smoothies & shakes, flavored beverages, nectars, fruit juices |
| Supplements | Encapsulated dietary supplements, gummies (soft chews) |
Properties of Alginate
| Physical Form | Powder |
| Color | White to yellowish-brown |
| Odor | No peculiar smell |
| Storage Temperature & Conditions | Room temperature in airtight container |
| Molecular Weight | Between 32,000 and 400,000 |
| Density | 1.0 g/cm3 |
| Viscosity (1% Sodium alginate) | 117 mPa s |
| Gelling Temperature | Optimum 37.6°C; can gel at room temperature |
| Melting Point | 99°C |
| Solubility | Solubility in cold water is slower and leads to obtaining a viscous solution |
| Claims (*Product Specific) | Natural*, Halal*, Kosher* |
Typical Formulations
Strawberry Gel Concentrate
Here is an example formulation of strawberry gel concentrate with sodium alginate and the percent composition of ingredients.
| Ingredient | % Composition |
| Sugar | 81.902 |
| Sodium alginate | 10.772 |
| Gum blend | 0.734 |
| Malic acid | 0.319 |
| Fumaric acid | 0.383 |
| Citric acid | 1.149 |
| Sodium citrate | 2.426 |
| Dicalcium phosphate | 0.575 |
| Artificial strawberry flavor | 1.452 |
| FD&C Red #40 | 0.003 |
| Maltodextrin | 0.284 |
The resulting mixture may spontaneously thicken and/or gel upon mixing with water to create a tasty, flavored dessert. The gel formulations may be optimized for mixing with cold or room temperature water with no specific need for hot water or boiling.
Source: Google Patents
Low-Fat Mayonnaise
Here is an example formulation of low-fat mayonnaise with sodium alginate and the percent composition of ingredients.
| Ingredient | % Composition |
| Oil | 15 |
| Egg yolk | 10 |
| Water | 29 |
| Vinegar | 4.5 |
| Sugar | 2 |
| Salt | 1.5 |
| Soy protein isolate | 1 |
| Sodium alginate | 38 |
Alginate biopolymer is used as a thickening and gelling agent due to its irreversible gelation property when combined with ionic salts such as calcium chloride.
Source: MDPI
Hypoallergenic Wheat Noodles
Here is an example formulation of hypoallergenic wheat noodles with sodium alginate, along with the composition of ingredients.
| Ingredient | Composition (g) |
| Hypoallergenic wheat flour | 24 |
| Sodium alginate | 0.6 |
| Salt | 0.6 |
| Starch | 6.0 |
| Curdlan | 0.3 |
| Water | 18 |
In hypoallergenic wheat flour (HWF), enzymes partially hydrolyze gluten. Adding sodium alginate results in harder noodles and decreases in rupture stress.
Source: J-STAGE
Alginate Formulation Considerations
| Physical Forms | Powder |
| Stability | – Temperature: Alginate generally forms thermostable gels over the range of 0-100°C. Above 100-120°C, alginate gels undergo depolymerization. – pH: Stable over a wide pH range – Moisture: Hygroscopic |
| Dosage | 1-5% or more, depending on the application and desired effect |
Alginate Grades & Variants
- Alginate Salts: Alginic acid can form salts with various cations, resulting in different properties and applications. Some of the commonly used salts of alginic acid are described in the table below:
| Salt | Details |
| Sodium Alginate | This salt forms a gel with calcium ions, making it useful for applications such as food thickening and encapsulation of flavors and drugs. |
| Potassium Alginate | This salt is similar to sodium alginate but has a slightly different gelation behavior. It is used in the food industry as a thickening and stabilizing agent. |
| Calcium Alginate | This salt is formed by combining sodium alginate with calcium ions. It forms a gel that is more stable than sodium alginate gel. It is used in the food industry for making gel-like products, such as fruit jellies and desserts. |
| Ammonium Alginate | This salt is formed by combining sodium alginate with ammonium ions. It is less commonly used in the food industry. |
- Difference in Alginates Based on the Seaweed Source: Alginate derived from different brown seaweed species will contain different proportions and sequences of M and G residues. These differences determine the alginate’s molecular weight, physical properties, and derived structures. Both molecular mass and molecular structure significantly affect the properties of gels formed by alginate.
- Modified Alginates: Alginates are exploited or synthesized for various biomedical applications by introducing different hydrophilic moieties, such as alkyl groups or hydrophilic polymers, in the alginate matrix. Long-chain alkyl groups, such as dodecyl or octadecyl, are bonded with alginates matrix via esterification. The various properties, such as rheology, gelling, and crosslinking characteristics, can be altered by modifying these.
Rheological Properties
Alginate gel particles are viscoelastic soft particles that can be deformed in response to external stimuli due to the presence of water in their gel network.
Alginates in solution produce pseudoplastic (shear-thinning behavior) liquids. In a study with a constant increase in the shear stress, alginate solutions with different concentrations demonstrated typical behavior of the non-Newtonian flow type, especially in the range of higher shear rates. This behavior occurs due to the alignment of the molecules in the colloidal solution.
Intrinsic Parameters Affecting the Rheological Properties of Alginates
Molecular Weight
| Effect on Properties | Reason |
| Gel Strength: Low molecular weight alginate creates a rigid gel structure. | The faster availability of shorter chains during gelling is likely why gel rheology depends on molecular weight. |
| Syneresis: Low molecular weight alginate gel has an increased tendency for syneresis. | A rigid gel structure resists the forces of deformation. |
Molecular Structure (M Blocks and G Blocks)
| Effect on Properties | Reason |
| Gel Strength: Brittlness is common in high G-block gels, while a high M-block gel will be more elastic. | G sequences form junctions with divalent cations. |
| Syneresis: Synerseis is higher with a greater proportion of alternating MG-blocks. | A high M-block content produces medium-strength gels with lower tendencies for syneresis. |
| Heat Stability: Alginates with high levels of mannuronic acid residues were far less heat stable. | G sequences form junctions with divalent cations. |
Particle Size
| Effect on Properties | Reason |
| Gel Strength: Higher rates of gelation and a lower final storage modulus result from using smaller particles, whereas larger particle size results in lower gelling rates and higher gel elasticity. | A change in the amount of total particle surface area causes these changes. Smaller particle sizes bring about a higher rate of calcium ion release and result in a lower gelation half-time. |
Process Parameters Affecting Rheological Properties of Alginate
Concentration
| Effect on Properties | Reason |
| Mechanical Strength: Mechanical strength decreases with a decrease in the concentration. | With concentration, the alignment of molecules in a colloidal solution changes. Also, an inadequate number of G-blocks are available for binding in a low alginate concentration solution. |
Temperature
| Effect on Properties | Reason |
| Rupture Strength: Rupture strength increases with a decrease in the gelation temperature. | Lowering gelation temperature slows the calcium diffusion rate in alginate gel which yields a more regular internal structure. |
| Gel Stability: M-block-rich alginate gel is less stable under heating conditions than G-block residues. | G sequences form junctions with divalent cations. |
pH
| Effect on Properties | Reason |
| Rheology: The pH value of the native alginate solution is close to 7.0 and has a Newtonian behavior. Shear thinning increases with a drop in pH. Below pH 3.0, alginic acid precipitates. | pH of the solution is brought down below the disassociation constant (pKa) of the polymer. |
| Shape of Beads: A decrease in pH increases the aspect ratio (ratio of radiuses of oblate particles). | The balance between the polymer viscosity and the interfacial tension determines the alginate particle shape. A lower pH gelation solution gives rise to a lower interfacial tension. |
| Gel Strength: Gel strength increases with an increase in pH | Carboxyl groups that are protonated form a more compact gel network due to the reduced electrostatic repulsion between alginate polymers. |
| Pore Size of Alginate Beads: In a low pH environment, gel particles shrink and pore size decreases, and vice versa. | At a pH above 4.4, the –COOH group ionizes and the negative charge increases the electrostatic repulsion leading to the expansion of the polymer chain and the swelling of the hydrophilic matrix. |
Cross-Linking Agent
| Effect on Properties | Reason |
| Strength: Strength increases with an increase in the concentration of cross-linking agents. Excess Ca ions increased gel strength for alginate with molecular weight (Mw) up to 150 kDa, after which the effect was negligible. | The gel-forming ability of alginate relies on binding G-blocks to divalent cations. |
| Shape of alginate beads: The crosslinking process creates a three-dimensional network structure that gives the beads stability and prevents them from easily deforming or losing their shape | Alginate particle shape is tailored by balancing the polymer viscosity and the interfacial tension. Viscosity is affected by cations. |
| Release Kinetics: Lower concentrations of cross-linking agents increase the release rate. | Lower concentrations of both alginate and divalent cations resulted in a flattened oblate shape with a higher surface area. |
| Pore Size: Alginate gels formed with Ba2+ or Al3+ have a smaller pore size than calcium alginate gels. | Depends on the affinity of the cation to the G-block. |
Gelling Phenomena
Gelatin forms translucent, opaque gels with uniform consistency. A study revealed that the consistency of alginate gels differs by concentration. Gelling in sodium alginate occurs at concentrations higher than 1-1.5%. Gels at concentrations of 0.5-1 are almost liquid and with low viscosity. Gels above this concentration are gelatinous and viscous.
The formation of alginate gels can be achieved by two methods: ionic crosslinking with cations (ionic gels) or acid precipitation (acidic gels). Compared to other polysaccharides such as gelatin or agar, alginate can form gel independent of temperature.
Ionic Alginate Gels
- Alginate forms gels by interacting with divalent cations. The binding of divalent cations is a highly selective process, and an alginate’s affinity to a cation increases in the order of Mn < Zn, Ni, Co < Fe < Ca < Sr < Ba < Cd < Cu < Pb.
- The “egg-box” model conventionally describes the ionotropic gelation of sodium alginate with calcium cations. In this model, calcium cations interact with guluronic acid monomers in the cavities formed by pairing the G sequences of the alginate molecular chains. Each cation binds with four G residues in the egg-box formation to form a 3-D network of these interconnected regions.
- Alginate affinity towards cations directly depends on the amount of G-blocks present in the alginate structure.
- Calcium is the most commonly used cation for crosslinking in the alginate gels. A major drawback of ionic alginate gels is that they can be destabilized by Ca chelators such as citrates, phosphates, carbonates, and lactates.
- Alginate can also gel in the presence of trivalent cations such as Al and Fe. The binding of trivalent cations forms a three-dimensional bonding structure that results in a more compact gel network.
Acidic Alginate Gels
- Alginic acid gels are formed when the pH of the solution is brought down below the polymer’s disassociation constant (pKa). M and G residues have pKa of 3.38 and 3.65, respectively. For this reason, alginate is negatively charged across a wide range of pH levels.
- A rapid decrease in pH results in precipitation of alginic molecules in the form of aggregates. A slow and steady drop in pH results in the formation of a continuous alginic acid bulk gel.
- Acid gels of alginate are stabilized by hydrogen bonding, and M-block residues have been shown to play a part in gelation.
Encapsulation Phenomena
The potential of the alginate to cause gelation upon contact with multivalent ions is valuable for preparing beads for encapsulating actives. These actives may include minor nutrients or other molecules with health benefits. Alginate can also be used to encapsulate bacteria and enzymes for the biotechnological production of molecules.
To optimize the delivery of actives inside the desired environment, the porosity of the encapsulation matrix and the release kinetics must be streamlined as described below.
- Porosity: The porous nature of alginate gel allows the substrate to diffuse in or out of the gel particles and is essential for immobilization. Small soluble molecules like glucose and insulin can diffuse in and out of alginate beads. The pore size of alginate gels is in the range of 5-200 nm.
- Release Behavior: Diffusion or erosion releases Core materials from alginate gel particles. Solvent-soluble low molecular weight active ingredients, such as drugs, vitamins, and sugars, smaller than the alginate gel’s pore size can diffuse in and out of the gel particles freely. Core materials are released by erosion when the alginate gel matrix disintegrates. Gel disintegration occurs at high pH or in the presence of cation chelators such as EDTA and citrate.
Safety and Regulatory Considerations
| FDA Information | In the United States, sodium alginate, calcium alginate, ammonium alginate, and potassium alginate are considered Generally Recognized as Safe (GRAS) by the FDA. The FDA has set specific limits on the amount of these salts used in different food categories. |
| EU Information | In the European Union, sodium alginate, calcium alginate, ammonium alginate, and potassium alginate are regulated by the European Food Safety Authority (EFSA). The EFSA has established an acceptable daily intake (ADI) for them. |
Health Effects of Alginate
- Digestive Health: Alginate has been found to affect digestive health positively. It can help promote regular bowel movements and prevent constipation. Alginate works by increasing the bulk and water content of the stool, making it easier to pass.
- Weight Management: Alginate has been studied for its potential role in weight management. It has been found to increase feelings of fullness and reduce appetite, which can help control calorie intake and promote weight loss.
- Heart Health: Alginate has been shown to lower triglycerides. It can bind to triglycerides and prevent their absorption into the bloodstream, thereby reducing cholesterol levels.
- Blood Sugar Control: Alginate has been found to benefit blood sugar levels. It can slow down the digestion and absorption of carbohydrates, resulting in a slower and more gradual release of glucose into the bloodstream. This can help prevent blood sugar spikes and maintain stable blood sugar levels.
Safety & Toxicity of Alginate
Alginate is generally considered safe for consumption and use in various applications. It is classified as a Generally Recognized as Safe (GRAS) ingredient by the U.S. Food and Drug Administration (FDA).
Alginate is not known to be toxic or harmful when consumed in normal amounts.
Identification Numbers
| Salt of Alginic Acid | Sodium Alginate | Potassium Alginate | Calcium Alginate | Ammonium Alginate |
| CAS Number | 9005-38-3 | 9005-36-1 | 9005-35-0 | 9005-34-9 |
| EC Number | 618-415-6 | 920-986-4 | 618-413-5 | 618-411-4 |
| INS No. (Food Additive) | INS 401 | INS 402 | INS 404 | INS 403 |
| E Number (Food Additive) | E 401 | E 402 | E 404 | E 403 |
| FEMA Number | 2015 | 2015 | 2015 | 2015 |
Acceptable Limits or Maximum Usage
Acceptable daily intake (ADI) for sodium alginate, potassium alginate, calcium alginate, and ammonium alginate are recorded as “not specified” by the JCEFA.
The maximum usage level of alginate in the food industry per the FDA is as follows:
Sodium Alginate
| Category of Food | Maximum Level of Use in Food as Served (percent) |
| Condiments and relishes | 1.0 |
| Pimento ribbon for stuffed olives | 6.0 |
| Confections and frostings | 0.3 |
| Gelatines and puddings | 4.0 |
| Hard candy | 10.0 |
| Processed fruits and fruit juices | 2.0 |
| All other food categories | 1.0 |
Potassium Alginate
| Category of Food | Maximum Level of Use in Food as Served (percent) |
| Confections and frostings | 0.1 |
| Gelatines and puddings | 0.7 |
| Processed fruits and fruit juices | 0.25 |
| All other food categories | 0.01 |
Ammonium Alginate
| Category of Food | Maximum Level of Use in Food as Served (percent) |
| Confections, frostings | 0.4 |
| Fats and oils | 0.5 |
| Gelatines, puddings | 0.5 |
| Gravies and sauces | 0.4 |
| Jams and jellies | 0.4 |
| Sweet sauces | 0.5 |
| All other food categories | 0.1 |
Calcium Alginate
| Category of Food | Maximum Level of Use in Food as Served (percent) |
| Baked goods | 0.002 |
| Alcoholic beverages | 0.4 |
| Confections and frostings | 0.4 |
| Egg products | 0.6 |
| Fats and oils | 0.5 |
| Gelatines, puddings | 0.25 |
| Gravies and sauces | 0.4 |
| Jams and jellies | 0.5 |
| Sweet sauces | 0.5 |
| All other food categories | 0.3 |
Fun Facts About Alginates
- Chefs and food scientists use alginate in a process known as spherification, a technique in molecular gastronomy. In this process, liquid foods are encapsulated in spheres or bubbles to create unique textures and presentations.
- Alginate is suitable for encapsulating living cells in a gel matrix, making it valuable in cell encapsulation and transplantation in the field of regenerative medicine.
Additional Resources
- Taylor & Francis Online – Critical Review on Magnesium Stearate
- PubMed Central – Article on Magnesium Stearate
- PubMed Central – Article on Magnesium Stearate and Tablet Manufacturing
- DOI Link – Study on Magnesium Stearate
- The Pharma Journal – Research Paper on Magnesium Stearate (PDF)
- ScienceDirect – Article on Magnesium Stearate
- Journals UMCS – Article on Alginates
- IntechOpen – Chapter on Alginates
- De Gruyter – Article on Alginates
- NCSU Repository – Thesis on Alginates (PDF)
- PubMed Central – Article on Alginates
- ScienceDirect – Article on Alginates



