Tartaric acid is a naturally occurring organic acid found in various fruits, plants, and wine. It’s used commercially in the food industry as a flavoring agent and an additive. Tartaric acid exhibits a slightly astringent and refreshing sour taste that contributes a strong tart flavor to food and beverages.
What is Tartaric Acid?
The chemical name of tartaric acid is 2,3-dihydroxybutanedioic acid, and its chemical formula is C4H6O6. It is a diprotic acid acting as a weak acid in solution. Tartaric acid is present in the form of 3 stereoisomers: dextro, levo, and meso. Dextro and levo are optically active, and meso is optically inactive.
Although tartaric acid was first isolated by the alchemist, Jabir Ibn Hayyan, Swedish chemist Carl Wilhelm Scheelea was credited for its discovery in 1769. Experiments with tartaric acid date back to the Romans and the Greeks, who observed “tartar” as a partially purified form of acid.
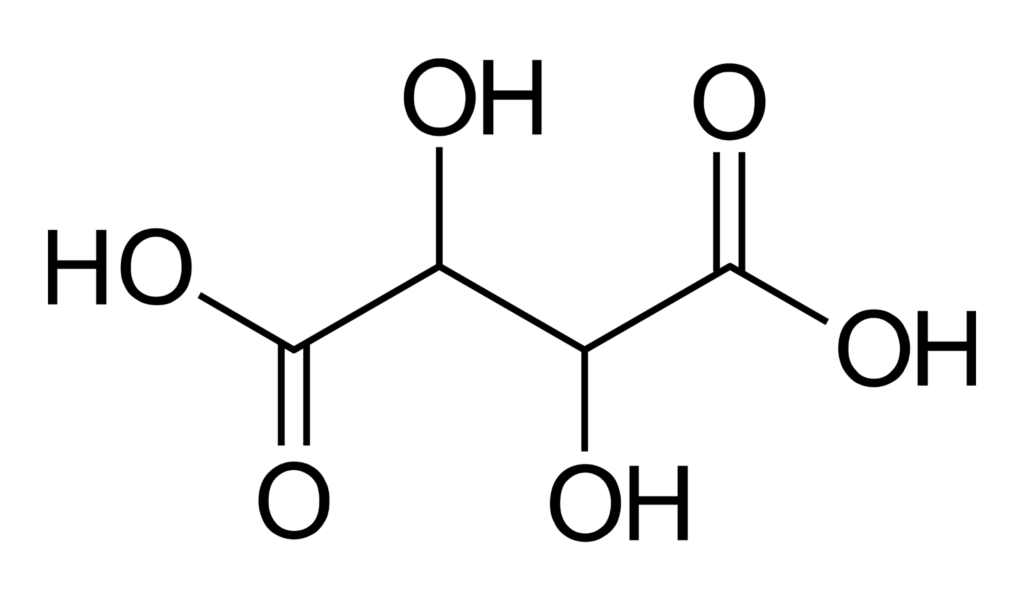
Chemical structure of tartaric acid. Source: Wikipedia
How is Tartaric Acid Produced?
Tartaric acid naturally occurs in plants like grapes, apricots, apples, bananas, avocados, and tamarinds. It can be isolated from these sources. Also, it is one of the primary acids found in wine. It is produced commercially by chemically treating potassium tartrate, a by-product of the wine industry.
Use of Tartaric Acid in the Food and Beverage Industry
Tartaric acid is added to foods to give them a tart and tangy flavor. It also acts as an acidulant in multiple food applications, lowering the pH of food products. This functionality also contributes to its preservative capabilities. In baking, tartaric acid is used to impart leavening to baked goods. It is also used in candy and frozen desserts, as well as in non-alcoholic and alcoholic beverages and confectionery items.
Tartaric acid is an essential additive in wine due to its prominent role in maintaining its chemical stability and color. It is used to prepare more balanced wines from a taste perspective, resulting in an increase in acidity and a decrease in pH content. It lowers the pH of fermenting wort to prevent bacteria in brewing as well and acts as a preservative after fermentation.
Industry Applications in the Food Industry
Tartaric acid is a multipurpose food additive that serves a variety of functions, including:
- Acidulant
- Flavoring agent
- Preservative (antimicrobial agent)
- Flavor enhancer
- Raising agent
- Sequestrant
- Antioxidant
- pH control agent
| Function | Applications |
| Acidulant, Preservative, Flavor Enhancer | Powder mixes, Jams, Jellies, Sauces |
| Acidulant, pH Regulator, Flavor Enhancer | Candies, Gummies, Soft Chews, Frozen Desserts |
| Leavening agent, Acidulant, Flavor Enhancer, Antioxidant | Cake, Bread |
| Acidulant, Flavor Enhancer, Effervescent | Carbonated Beverages, Acidified Beverages, Fruit Juices, Marmalades, Carbonated Water |
| pH Regulator, Stabilizer | Wine, Brewing |
Product Examples
| Type | Examples |
| Convenience Foods | Powder Mixes, Jams, Jellies, Sauces |
| Confectionery | Candies, Gummies, Soft Chews, Frozen Desserts |
| Bakery | Cake, Bread |
| Alcoholic Beverages | Wine, Brewing |
| Non-Alcoholic Beverages | Carbonated Beverages, Acidified Beverages, Fruit Juices, Marmalades, Carbonated Water |
Properties of Tartaric Acid
| Molecular Weight | 150.09 g/mol |
| Melting Point | 171°C |
| Density | 1.79 g/cc |
| Appearance | Crystalline white solid |
| Solubility | Freely soluble in water and ethanol |
| Heat of Solution | -14.42 KJ/mol |
Typical Formulations
Candy
A dry, free-flowing candy mix powder was prepared from the following components. Tartaric acid is used as a flavoring agent in this formulation. The resulting product is a relatively dry and crumbly mix. The mix is then heated at 250° F for 30 minutes or until all ingredients are melted. The resulting candy melt is then cooled in a refrigerator.
| Ingredient | % Composition (Sugar Basis) |
| Fructose | 100 |
| Tartaric Acid (alone or in combination with other acids) | 3.0 |
| Color | 0.02 |
| Flavor (fruit flavors) | 0.2 |
| Propylene glycol | 1 |
Source: Google Patents
Effervescent Powder
An effervescent powder that can be used for obtaining an instant sparkling beverage was made according to the following formulation. The resulting product can be used in powder, granule, or tablet form — it self-cools and releases gas when dissolved in liquids suitable for ingestion, for example, water, natural fruit juices, etc. The formulation may contain other additives, like sweeteners, sugars, anti-humectants, colorants, flavorings, etc.
| Ingredient | Parts |
| Sodium Bicarbonate | 2 |
| Citric Acid | 1 |
| Tartaric Acid | 3 |
Source: Google Patents
Jelly
Papaya jelly was made using papaya fruit pulp and the following components.
| Ingredient | % Pulp Basis |
| Papaya Pulp (adding sugar/ liquid glucose)adjusted to 80-81 TS | 100 % |
| Citric Acid: Malic Acid: Tartaric Acid (40:35:25) | 1.2 % |
| Sodium citrate: Sodium Tartrate (1:1) | 0.3 % |
Source: Research Gate
Tartaric Acid Formulation Considerations
Tartaric acid’s properties have several important implications for the formulation, as described in the table below.
Physical Forms
The pKa values for tartaric acid are: pKa1=2.98 and pKa2=4.34. The 1 mM solution of tartaric acid has a pH of 3.18. Adding tartaric acid to food products lowers the system’s pH, imparting many other benefits, including increased shelf life. By lowering the pH of a solution, the tartaric acid acts as an effective antimicrobial agent by creating an environment too acidic for most microorganisms to grow.
Dosage
The dosage of tartaric acid in formulations ranges from 0.05% to 0.5% (or more), depending on the product and the desired effect. However, it is generally used with other acids, such as citric acid and malic acid, to obtain the desired flavor profile as an additive in food products.
Effects on pH Properties
The pKa values for tartaric acid are: pKa1=2.98 and pKa2=4.34. The pH of 1 mM solution of tartaric acid has a pH of 3.18. The addition of tartaric acid to food products lowers the pH of the system imparting many other benefits including increased shelf life. By lowering the pH of a solution, the tartaric acid acts as an effective antimicrobial agent by creating an environment too acidic for most microorganisms to grow.
Effects on Shelf Life
Tartaric acid provides multiple benefits in terms of shelf life, including increased shelf stability, color stability, and preservation. Tartaric acid acts as a chelating agent and produces canned fruit products. Chelates are formed when an organic acid binds with a metal, preventing its reaction with another chemical. Chelating agents prevent enzymatic browning by forming a complex free metal and inhibitors through an unshared pair of electrons in their molecular structures.
Effects on Sensory Properties
Tartaric acid functions as a sequestrant. It aids in its activity as an antioxidant. It has also been reported to have anti-hypertensive potential. Tartaric acid and dietary fibers have been reported to synergistically improve digestive health. It was reported to show antihyperglycemic and antidyslipidemic effects and improve glucose tolerance.
Biological Activity
Tartaric acid exhibits a slightly astringent and refreshing sour taste. It has a more robust, sharper taste than citric acid. Tartaric acid hits the tip and front quarter of the tongue. It is expected to give the classic overt, mouthwatering, and jaw-clenching experience often described as acidity in wine.
Sources
The pKa values for tartaric acid are pKa1=2.98 and pKa2=4.34. The 1 mM solution of tartaric acid has a pH of 3.18. Adding tartaric acid to food products lowers the system’s pH, imparting many other benefits, including increased shelf life. By lowering the pH of a solution, the tartaric acid acts as an effective antimicrobial agent by creating an environment too acidic for most microorganisms to grow.
Comparison with Other Acidulants
Tartaric acid has the edge over other acidulants for food applications because it is a natural acid with an astringent flavor. As a diprotic acid, it can donate two protons per molecule, making it more effective as an acidulant than monoprotic acids. Tartaric acid is the most water-soluble of the solid acidulants.
Generally, tartaric acid is used in smaller doses than other acidulants, as it is more potent. For example, citric acid is usually used in a 1-2% concentration, while tartaric acid is used in a 0.1-0.2% concentration.
Safety & Regulatory Considerations
| FDA Information | L (+) -tartaric acid (E334) is a food additive in the EU. The Scientific Committee for Food established an acceptable daily intake of 30 mg/Kg body weight/day. |
| EU Information | In the EU, L (+) -tartaric acid (E334) is a food additive. The Scientific Committee for Food established an acceptable daily intake of 30 mg/Kg body weight/day. |
Safety & Toxicity of Tartaric Acid
Overconsumption could lead to increased thirst, vomiting, diarrhea, abdominal pain, and gastrointestinal inflammation.
Identification Numbers
| CAS Number | L (+) tartaric acid: 87-69-4D (-) tartaric acid: 147-71-7Mesotartaric acid: 147-73-9 |
| EC Number | 201-766-0 |
| E No. (Food Additive) | E 334 |
| INS No. (Food Additive) | INS 334 |
| JECFA Number | 621 |
Fun Facts About Tartaric Acid
- Tartaric acid has been used for centuries in winemaking. The ancient Greeks and Romans were aware of its presence in wine sediments, known as tartar, and used it for medicinal purposes.
- Tartaric acid may appear as “wine diamonds” found in the cork of a wine bottle.
- Tartaric acid is used to make solutions for determining glucose in the medical industry.
- Tartaric acid has been used in traditional medicine for its laxative properties. It is sometimes included in pharmaceutical formulations and oral care products.
Additional Resources
- USDA – Tartaric Acid Report 2011
- SMEA Srl – Tartaric Acid Production Plant
- FDA – Title 21 – Part 184 – Subpart B – Tartaric Acid
- FDA – Title 21 – Part 184 – Tartaric Acid
- WHO Food Additives Database – Tartaric Acid
- Google Patents – WO2007147226A1 – Tartaric Acid Production Process
- Google Patents – US3957579A – Process for Producing Tartaric Acid
- ScienceDirect – Organic Acids: Tartaric Acid
- Aqion – pH of Organic Acids
- Semantic Scholar – Fruit Juice Processing Technology
- AGRIS – Tartaric Acid in Fertilizer Industry
- QASCF – Effect of Tartaric Acid on Wine Quality
- NCBI – Tartaric Acid: A Potential Nutraceutical Agent
- WakaWaka Wine Reviews – Doing an Acid Tasting: Thinking Briefly about Tartaric and Malic Acids
- ResearchGate – Effect of Tartaric Acid and Dietary Fibre from Sun-Dried Raisins on Colonic Function and on Bile Acid and Volatile Fatty Acid Excretion in Healthy Adults
- PubMed – Tartaric Acid: A Key Molecule in Medicinal Chemistry Research









