Hydrobromic acid’s strength and unique characteristics have established it as a critical element in numerous applications, from synthesizing bromine-containing compounds to manufacturing pharmaceutical products. A strong acid characterized by a sharp, acrid odor and corrosive properties, it has a simple diatomic structure. It is renowned for its high reactivity and polarity, making it a compelling choice for product developers across many industries.
What is Hydrobromic Acid?
Hydrobromic acid (HBr), or hydrogen bromide, is a potent mineral acid constituted by hydrogen and bromine. With a pH typically ranging from 0.4 to 1, it is a colorless liquid emitting a pungent, acrid odor and is remarkably soluble in water. As a binary acid, hydrobromic acid comprises only two elements, exhibiting properties that closely align with those of hydrochloric acid. Its pure form, which is exceedingly reactive, can pose handling hazards. The acid is frequently employed as a reagent in chemical synthesis and catalyzes organic reactions. Its applications extend to the production of pharmaceuticals like brompheniramine, and it acts as a bromine source in fabricating dyes, insecticides, and flame retardants.
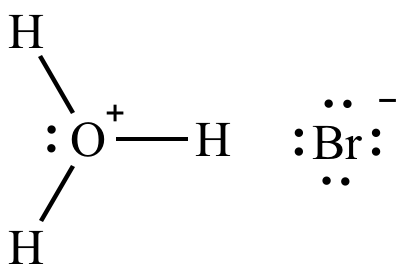
Lewis structure of hydronium bromide.Source: Wikipedia
How is Hydrobromic Acid Produced?
Hydrobromic acid does not naturally exist as a pure substance due to its high reactivity and readiness to react with water, forming a hydrated form known as a hydrobromic acid solution. However, bromide ions (Br–) can naturally occur in seawater, certain sedimentary rocks, and various minerals. Hydrobromic acid can form as a byproduct when these substances interact with potent acids, such as sulfuric acid, hydrochloric acid, or phosphoric acid.
Hydrobromic acid can be industrially manufactured via several methods, including:
- The reaction of bromine with sulfur or phosphorus and water: The most prevalent industrial method for hydrobromic acid production. Here, bromine reacts with either sulfur or phosphorus to generate hydrogen bromide gas (HBr), subsequently dissolved in water to create hydrobromic acid.
For instance, bromine (Br₂) reacts with sulfur dioxide (SO₂) and water (H₂O), yielding hydrogen bromide (HBr) and sulfuric acid (H₂SO₄)—a reaction byproduct.
The balanced chemical equation is:
Br₂ + SO₂ + 2 H₂O → H₂SO₄ + 2 HBr
The hydrogen bromide gas generated in this reaction is then dissolved in water to create hydrobromic acid.
- Electrolytic production: Hydrobromic acid can also be generated through the electrolysis of a bromide solution. In this process, an aqueous hydrogen bromide solution can be manufactured by feeding bromine into an aqueous catholyte liquor and introducing an electrical current through the electrolytic cell.
Here, the catholyte liquor comprises water and a source of bromide ions, while the anolyte liquor contains an electrically conductive substance such as sulfuric acid. A diaphragm or membrane segregates the two liquors, preventing mixing. Bromine is fed into the catholyte and reacts with water to generate hydrobromic acid and oxygen gas when an electric current is introduced to the cell. The hydrobromic acid produced in the catholyte can be gathered as an aqueous solution.
- Treating bromides with non-oxidizing acids: Hydrobromic acid can also be prepared by reacting with non-oxidizing acids such as phosphoric or acetic acids.
When a bromide salt is treated with a non-oxidizing acid, such as phosphoric or acetic acid, hydrogen bromide gas is liberated. This gas can then be collected and dissolved in water to form hydrobromic acid.
- Reaction of sulfuric acid with potassium bromide: A displacement reaction occurs when sulfuric acid and potassium bromide are mixed, whereby the sulfate ion displaces the bromide ion. This reaction generates potassium sulfate and hydrogen bromide gas.
The balanced chemical equation for this reaction is:
KBr + H₂SO₄ → K₂SO₄ + 2HBr
Hydrogen bromide gas, produced in the reaction between sulfuric acid and potassium bromide, can be gathered and dissolved in water to create hydrobromic acid, resulting in an aqueous hydrobromic acid solution.
Once produced, hydrobromic acid is frequently used in situ—that is, it is employed immediately in the reaction it is intended for without undergoing further isolation or purification. This is because hydrobromic acid is highly reactive and tends to react with the container it is stored in over time, making long-term storage problematic.
Uses and Applications of Hydrobromic Acid
Hydrobromic acid, an incredibly potent mineral of hydrogen and bromine, has numerous industrial uses across various sectors. It’s primarily used as a reagent and catalyst due to its high reactivity and acidic solid nature. Its role is significant in producing both inorganic bromides and organobromine compounds, with applications in industries ranging from oil and gas drilling to pharmaceuticals. Additionally, its utility as a catalyst in organic synthesis is noteworthy, as is its application in etching and engraving, which brings precision to the design and fabrication of materials. Furthermore, its function in alkylation processes makes it invaluable in producing high-octane fuels. The following sections delve into this versatile chemical’s multiple uses and applications, underscoring its industrial importance.
- Production of Inorganic Bromides: Hydrobromic acid is crucial in synthesizing various inorganic bromides, such as sodium bromide, calcium bromide, and zinc bromide. These compounds have diverse applications in oil and gas drilling, pharmaceuticals, and more.
- Production of Organobromine Compounds: Hydrobromic acid is often utilized as a bromide ion source to hydrobromination alkenes, alkynes, and aromatic compounds. The resultant organobromine compounds find extensive use across numerous industries, including pharmaceuticals, agrochemicals, and materials science.
- A Catalyst for Organic Synthesis: Hydrobromic acid is a common catalyst in various organic synthesis reactions due to its ability to protonate organic compounds, activating them for the nucleophilic attack. It plays a part in manufacturing numerous pharmaceuticals, flavors, and fragrances, including analgesics, sedatives, and antihistamines.
- Etching and Engraving: Hydrobromic acid is applied in etching and engraving diverse materials. It can selectively etch or engrave the surface when applied to glass to create intricate designs, patterns, or text. It’s also employed in etching metals, especially those resistant to other acids. Hydrobromic acid (HBr) is integral to semiconductor production for etching silicon wafers, which are subsequently used to fabricate various electronic components and integrated circuits. The etching process selectively removes unwanted material from the wafer’s surface using HBr, facilitating precision shaping of the components.
- Alkylation Processes: Hydrobromic acid often acts as a catalyst in alkylation reactions, which is vital for producing high-octane gasoline. In these reactions, hydrobromic acid reacts with an alkene to form an alkyl bromide, which then reacts with an isoparaffin to create a branched-chain alkane with a higher octane rating. Oil refiners use anhydrous hydrobromic acid and other compounds, such as sulfuric acid, to produce high-octane gasoline and aviation fuels.
- Flame Retardants: Hydrobromic acid is a fundamental ingredient in producing certain fire retardant compounds, such as tetrabromobisphenol A (TBBPA), frequently used in electronics and other applications. These fire retardant compounds are added to fabrics, plastics, and other materials to reduce flammability and enhance public safety.
Properties of Hydrobromic Acid
| Chemical formula | HBr |
| Molecular weight (g/mol) | 80.91 |
| Appearance | Colorless to yellowish liquid or gas |
| Odor | Pungent and irritating |
| Boiling point (°C) | -66.8 |
| Melting point (°C) | -86.9 |
| Refractive index (at 20°C) | 1.423 |
| Density g/mL (for 48% aqueous solution) | 1.49 |
| Vapor Pressure (at 25°C) (mmHg) | 26000 |
| Solubility | Completely miscible with water |
| pH | Highly acidic (pH of 48% aqueous solution is about 0.5) |
| Reactivity | Strongly reactive with metals, metal oxides, and alkalis |
| Storage conditions | Hydrobromic acid should be stored away in a cool, dry, and well-ventilated area. It should be kept in a tightly closed container made of a material compatible with the acid, such as glass or high-density polyethylene. Hydrobromic acid should be kept separate from strong oxidizers, alkalis, and other incompatible materials. |
Safety & Regulatory Considerations
| USA | Hydrogen Bromide is included on the Right-to-Know Hazardous Substance List due to its recognition as a hazardous substance by key regulatory bodies, including OSHA, ACGIH, DOT, NIOSH, and NFPA. This compound is also categorized on the Special Health Hazard Substance List. |
Safety & Toxicity of Hydrobromic Acid
Hydrobromic acid (HBr), a potent and corrosive acid, necessitates careful handling due to its significant health and safety risks. The inhalation of its fumes can trigger respiratory irritation, prompting coughing and respiratory difficulties. Direct skin or eye contact with concentrated hydrobromic acid can lead to severe burns and tissue damage. This acid can inflict substantial damage to the gastrointestinal tract and other internal organs if ingested. As for environmental toxicity, hydrobromic acid threatens aquatic organisms if improperly disposed of and contaminates the environment due to its high water solubility. Therefore, the correct disposal of hydrobromic acid is essential.
Identification Numbers
| Chemical Name | Hydrobromic Acid |
| CAS Number | 10035-10-6 |
| REACH Registration Number | 233-113-0 |
Acceptable Limits or Maximum Usage
The maximum usage level of Hydrobromic Acid in {industry} per the {regulatory agency} is as follows.
| Category | Usage Level |
| OSHA | The legal airborne permissible exposure limit (PEL) is 3 ppm, averaged over an eight-hour work shift. |
| NIOSH | The recommended airborne exposure limit (REL) is 3 ppm, a value that should not be exceeded at any time. |
| ACGIH | The threshold limit value (TLV) is 2 ppm, a figure that should not be surpassed at any time. |
Fun Facts About Hydrobromic Acid
- The name “hydrobromic acid” stems from its chemical composition, which consists of hydrogen (H), bromine (Br), and a water molecule (H2O). This results in the chemical formula HBr, with the prefix “hydro,” indicating the presence of water.
- Hydrobromic acid is employed in gold extraction from geological materials. As a potent acid capable of dissolving metals, including gold, hydrobromic acid is utilized in a process that involves hydrobromic acid and bromine solution to dissolve gold from geological material. Subsequently, the gold is extracted using methyl isobutyl ketone.