Formic acid, or methanoic acid, is a standard carboxylic acid with the chemical formula HCOOH. This colorless, weak organic acid occurs naturally in various sources, such as ant venom and certain bacteria. Additionally, formic acid has wide-ranging applications and is frequently used in industries including textile dyeing, leather tanning, and rubber production. Owing to its uses as a preservative, disinfectant, and reducing agent in medicine, formic acid has been a vital topic of many research studies.
What is Formic Acid?
Formic acid is the simplest carboxylic acid, consisting of a carboxyl group (-COOH) attached to a hydrogen atom. It occurs naturally in insects, plants, and animals, including ants, nettles, and honeybees. However, it is predominantly produced synthetically on an industrial scale.
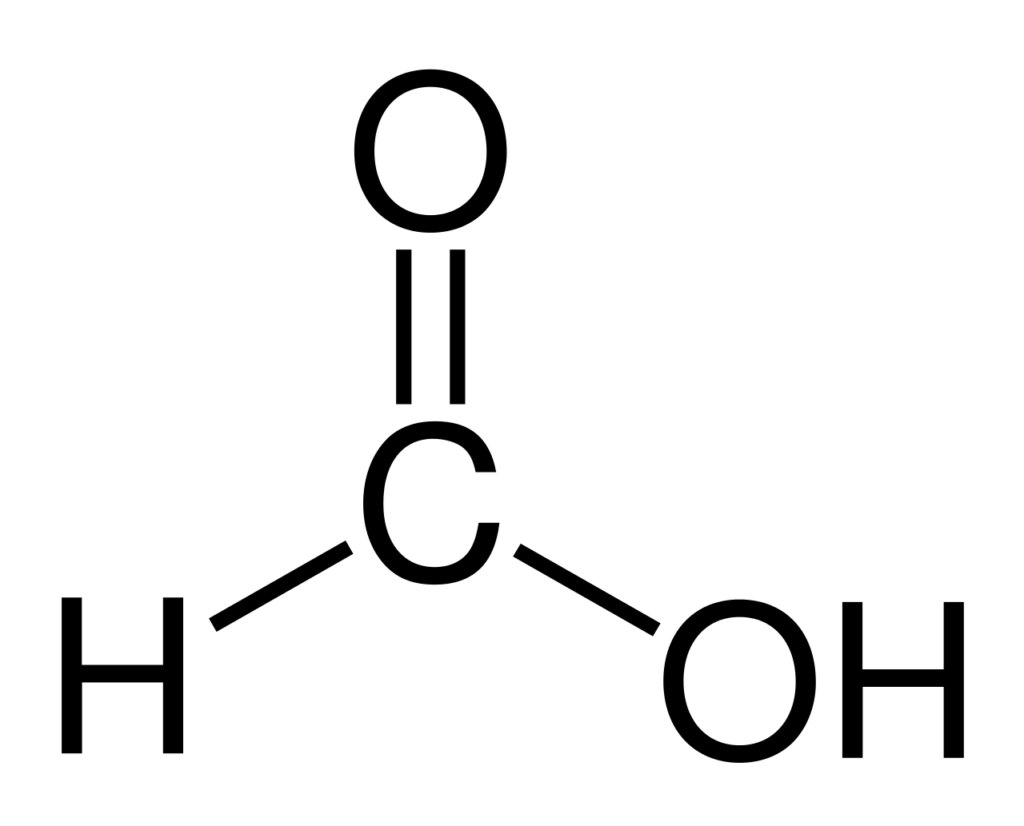
Source: Wikipedia
Formic acid serves several functions in nature. Certain species of ants, like the red imported fire ant (Solenopsis invicta) and the Argentine ant (Linepithema humile), produce formic acid for defense. Some plants, like stinging nettle (Urtica dioica) and hogweed (Heracleum mantegazzianum), produce formic acid to deter predators. Formic acid is usually extracted from these natural sources through distillation or extraction processes.
How is Formic Acid Produced?
Formic acid also serves as an essential organic acid in various industrial applications. Below are two standard methods for manufacturing formic acid:
Methanol Carbonylation Process: In this method, methanol reacts with carbon monoxide in the presence of a catalyst, typically rhodium or iodomethane, to produce formic acid.
CH₃OH + CO → HCOOH
This reaction occurs under high pressure (typically 30-50 bar) and around 200°C. The formic acid is then separated from the reaction mixture by distillation.
Methanol Oxidation Process: Here, methanol is oxidized using an appropriate oxidizing agent, such as air or oxygen, in the presence of a catalyst such as copper or silver. The oxidation reaction produces formaldehyde, which is then further oxidized to formic acid.
2 CH₃OH + O₂ → 2 HCHO + 2 H₂O
2 HCHO + O₂ → 2 HCOOH
This reaction occurs at around 150-200°C and around 1-2 bar pressure. The formic acid is then separated from the reaction mixture by distillation.
Please note that other methods exist for producing formic acid, but the above-mentioned processes are the most common.
Applications and Uses of Formic Acid
- Chemical Industry: Formic acid is a vital reducing agent used in various chemical reactions, thanks to the formate ion (HCOO−) capable of donating hydrogen atoms and electrons, thereby reducing other molecules.
Here are a few instances where formic acid acts as a reducing agent:
- Reduction of metal ions: It can decrease metal ions such as silver, gold, and platinum to their respective metal atoms, which is critical for creating metallic nanoparticles.
- Reduction of carbonyl compounds: It reduces carbonyl compounds, like aldehydes and ketones, for synthesizing alcohols. This process involves a nucleophilic addition of the formate ion (HCOO−) to the carbonyl group, succeeded by the protonation and reduction of the intermediate.
- Reduction of nitro compounds: It can lower nitro compounds like nitrobenzenes, nitroalkenes, etc., to their corresponding amines.
- Reduction of sulfur compounds: It can decrease sulfur compounds such as sulfides, sulfones, sulfoxides, and sulfides, converting them to their respective sulfurs.
- Dehalogenation reactions: Reductive dehalogenation can reduce halogenated compounds to matching hydrocarbons.
- Textile Dyeing: It is a common reducing agent used in textile dyeing. It enhances the colorfastness of certain dyes on wool and silk materials and aids in the dye’s fixation, leading to brighter and longer-lasting color.
- Leather Tanning: Formic acid is a staple in the leather tanning industry, where it’s used in the pickling process – the initial stage of tanning. Animal hides are soaked in a formic acid solution during pickling, lowering the pH and helping remove residual flesh, fat, or hair.
- Rubber Production: It is a coagulating agent for natural latex in rubber production. Lowering the pH destabilizes the colloidal suspension of rubber particles in the liquid, causing them to form a solid mass. Besides, it’s used as a preservative for rubber products and a cleaning agent for rubber processing equipment.
- Livestock Feed: Formic acid is a widely used preservative and antibacterial agent in livestock feed. It helps prevent bacterial and fungal growth, which can spoil the feed and reduce its nutritional content. Besides, it can enhance the feed’s digestibility, improving the animals’ health and productivity.
Derivatives of Formic Acid
Formic acid generates a range of derivatives, such as esters, amides, salts, and halides.
- Formic Acid Esters: Formic acid combines with alcohol to produce an ester and water. A strong acid like sulfuric acid catalyzes this reaction.
HCOOH + R’OH → HCOOR’ + H₂O, where R’ represents an alkyl group like methyl or ethyl.
Application: Formic acid esters serve as solvents in manufacturing artificial flavors.
- Formamides: Formic acid interacts with a primary amine to yield an amide and water, a reaction catalyzed by a potent acid such as phosphoric acid.
HCOOH + NH₂R’ → HCONHR’ + H₂O, where R’ stands for an alkyl or aryl group, like methyl or phenyl.
Application: Formamides are employed as solvents in producing pesticides and pharmaceuticals.
- Formate Salts: Formate salts result from the reaction of formic acid with metal hydroxides or carbonates. Sodium formate (HCOONa) and potassium formate (HCOOK) are examples.
HCOOH + M’OH → HCOOM’ + H₂O
HCOOH + M’₂CO₃ → 2HCOOM’ + CO₂, where M’ signifies a metal like Na or K.
Application: Formate salts are used in leather, textile, and paper production. They also function as a source of formic acid in the textile industry.
- Formyl Halides: Formyl halides are created through the reaction of formic acid with hydrogen halides, as demonstrated by formyl chloride (HCOCl) and formyl bromide (HCOBr).
HCOOH + HX → HCOX + H₂O, where X represents a halogen atom like Cl or Br.
Application: Formyl halides act as reagents in organic synthesis.
Properties of Formic Acid
| Chemical Formula | CH₂O₂ |
| Appearance at 25 °C | Colorless liquid |
| Odor | Pungent, penetrating odor |
| Density (g/cm³) at 25 °C | 1.22 |
| Molecular Weight/ Molar Mass (g/mol) | 46.03 |
| Boiling Point (°C) | 100.8 |
| Melting Point (°C) | 8.4 |
| Solubility | Miscible with water |
| pH Range (10% solution in water) | < 2 |
| Storage conditions | Keep tightly closed in a dry and cool place. Keep in properly labeled containers. Keep away from heat, sparks, flame, and other sources of ignition (i.e., pilot lights, electric motors, and static electricity). |
Formic Acid Formulation Considerations
Procedure Example: Pickling & Pre-tanning of Leather
| SI No. | Ingredient Name | % Wt. |
|---|---|---|
| 1 | Reductive saccharide with a dextrose equivalent of 10 to 100 | 30 – 55 |
| 2 | Aliphatic dialdehydes containing 2 to 8 carbon atoms | 2-75 |
| 3 | Phenol-sulfonate reaction product | 1-10 |
| 4 | Formic acid | 0-5 |
| 5 | Water | To make up 100 |
| Total | 100 |
Formulation Procedure
- Start by filling a pickling drum with water, generating a salt-free aqueous solution (termed Liquor A).
- Next, introduce the phenol-sulfonate reaction product to Liquor A, ensuring a molar ratio of phenol to SO3 ranging between 1:1.1 and 1:2.2.
- Add formic acid to Liquor A, adjusting the pH between 2.5 and 3.5. This assists in removing any lingering hair and proteins from the hide or skin. The required amount of sulfuric acid will vary depending on the drum size and the quantity of water utilized.
- Immerse the raw hides in Liquor A, allowing them to pickle for 24 to 48 hours.
- Once the pickling is completed, remove the hides from the drum and prepare a new aqueous solution called Formulation B.
- Add the reductive saccharide and the aliphatic dialdehyde to Formulation B.
- Return the pickled hides to the drum and introduce Formulation B into the same bath.
- Initiate the pre-tanning process on the pickled hides in the combined one-bath pickling/pre-tanning process, lasting between 4 and 6 hours.
- Following the pre-tanning stage, extract the hides from the drum, ensuring they are rinsed thoroughly with water.
This process enables the production of wet white leathers well-suited for subsequent processing using all conventional tanning methods. Moreover, the utilization of treatment baths devoid of neutral salts and heavy metals enhances the environmentally friendly nature of this process. Including formic acid in the pickling stage maintains a consistent pH level across the entire hide or skin, ensuring uniformity.
Safety & Regulatory Considerations
| FDA Information | Formic acid is cited in 21CFR186.1316 |
| EU Information | According to the harmonized classification and labeling (CLP00) approved by the European Union, Formic acid causes severe skin burns and eye damage. Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance is toxic if inhaled, is a flammable liquid and vapor, is harmful if swallowed, and causes severe eye damage. |
Safety & Toxicity of Formic Acid
Formic Acid is a corrosive and toxic compound that poses a significant risk of causing severe burns and tissue damage if it comes into direct contact with skin, eyes, or mucous membranes. The inhalation of formic acid vapors may lead to nose, throat, and lung irritation, potentially inducing coughing, wheezing, and breathlessness. Long-term exposure to formic acid vapors can also result in headaches, dizziness, and mental confusion. It’s crucial to note that formic acid is a flammable liquid with a flash point of 68 °C.
Ingestion of formic acid may induce nausea, vomiting, abdominal discomfort, and diarrhea. In extreme cases, it could result in metabolic acidosis, a potentially life-threatening condition in which the body produces excess acid, leading to organ damage and critical complications.
Formic acid’s acute toxicity is low, indicating that immediate harm from small doses is unlikely. However, extended or repeated exposure to formic acid can result in chronic toxicity, evidenced by skin irritation, respiratory issues, and potential damage to the liver and kidneys.
Identification Numbers
| Chemical Name | Formic Acid |
| CAS Number | 64-18-6 |
| EC Number | 200-579-1 |
Fun Facts About Formic Acid
- The name “formic” comes from the Latin word “formica,” which means ant. This is because formic acid was first isolated from the bodies of red ants & it is a significant component of the venom produced by the sting of the infamous bullet ant, which is known to have one of the most painful insect stings in the world.
- The European Space Agency’s Philae lander, which landed on a comet in 2014, detected the presence of formic acid on the comet’s surface.
- Researchers have also found that formic acid is a promising alternative to hydrogen as a fuel source for the fuel cell. Due to their high theoretical energy density, very high-power densities have been achieved in direct formic acid fuel cells.






