Aniline is a colorless, oily liquid with a distinctive odor and is used in various industries, including chemical manufacturing, pharmaceuticals, and the production of synthetic dyes and pigments.
What is Aniline?
Aniline is an organic compound with the chemical formula of C6H5NH2, also known as aminobenzene/benzenamine. It consists of an aromatic benzene ring and an amino (-NH2) group known as the simplest aromatic amine. Aniline is a colorless to brownish oily liquid with a distinctive amine odor. It is slightly soluble in water but highly soluble in most organic solvents.
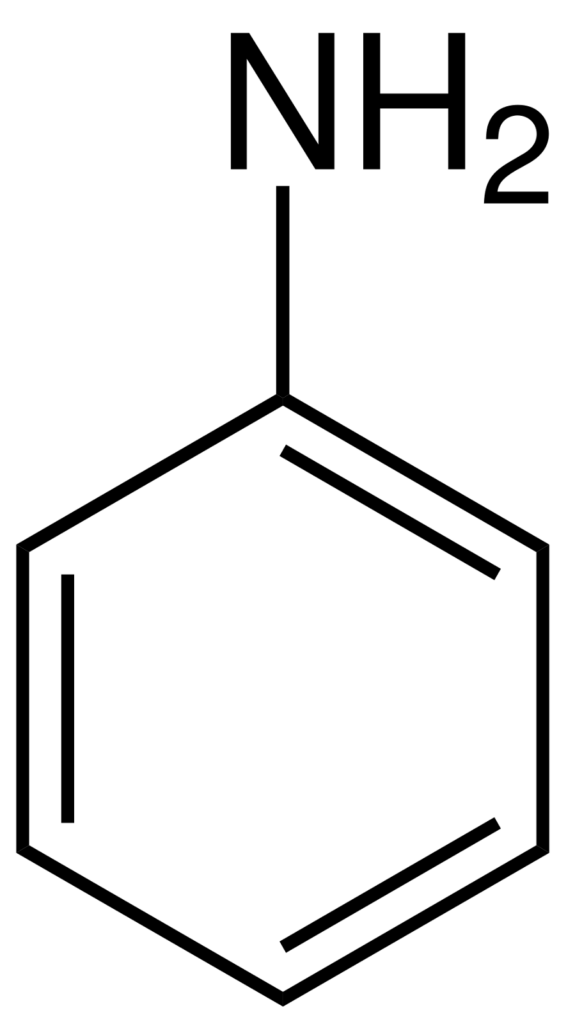
Source: Wikipedia
One of the most notable features of aniline is its versatility in chemical reactions. It is an essential precursor to various chemical compounds, including pharmaceuticals, dyes, rubber processing chemicals, and herbicides. Its use as a precursor is due to its ability to undergo a wide range of reactions, such as acylation, alkylation, and diazotization.
Aniline is also a crucial chemical intermediate in the production of many organic compounds. Its chemical reactivity as a nucleophile and its ability to undergo electrophilic substitution, oxidation, and reduction reactions make it an important chemical that is extensively used in industrial and commercial applications for over a century.
How is Aniline Produced?
Aniline, known as phenylamine, is an organic compound not commonly found in nature. It is typically produced synthetically through various industrial processes. However, small amounts of aniline can be found in some natural sources. For example, aniline has been detected in the roots of some plant species, such as Vicia Faba.
Aniline is also produced by specific microorganisms capable of breaking down aromatic hydrocarbons found in petroleum, coal tar, or other industrial wastes as a byproduct.
There are several methods of aniline production, some of which include:
| Reduction of Nitrobenzene | The most common method of aniline production, in which nitrobenzene is reduced to aniline using iron or iron oxide as a catalyst. C₆H₅NO₂ + 6H → C₆H₅NH₂ + 2H₂O The reaction takes place in the presence of a reducing agent such as tin, zinc, or hydrogen gas. |
| Catalytic Hydrogenation of Nitrobenzene | In this method, nitrobenzene is hydrogenated in the presence of a catalyst such as nickel or palladium to produce aniline. C₆H₅NO₂ + 3H₂ → C₆H₅NH₂ + 2H₂O |
| Amination of Benzene | Benzene is reacted with ammonia in the presence of a catalyst such as iron oxide or platinum to produce aniline. C₆H₆ + NH₃ → C₆H₅NH₂ + H₂ |
| Ammonolysis of Phenol | This process involves the reaction of phenol with ammonia in the presence of a catalyst, such as iron or copper. The reaction produces aniline along with water as a by-product. C₆H₅OH + NH₃ → C₆H₅NH₂ + H₂O |
Use of Aniline in Various Industries
In the chemical industry, aniline is used as a starting material for producing various other chemicals, including polyurethane foams, rubber, and pesticides. Aniline is also used to produce pharmaceuticals, such as antimalarial drugs and analgesics like paracetamol. Additionally, aniline is a critical ingredient in producing synthetic dyes and pigments in textiles, paints, and inks.
Applications Across Industries
| Industry | Applications |
| Dye and Pigment | It is used as a precursor, a chain extender, and a cross-linking agent in producing various polymers. As a precursor, aniline produces polyurethanes, polyamides, and other copolymers, which are widely used in various applications, including insulation, adhesives, and coatings. As a chain extender, aniline is used to improve the properties of specific polymers. For example, aniline can be used as a chain extender in producing polyurethanes to increase their tensile strength and tear resistance. As a co-curing agent, aniline is combined with other curing agents, such as diaminodiphenylsulfone (DDS) or dicyandiamide (DICY), in the production of epoxy resins to cross-link the polymer chains and form highly durable and heat-resistant materials. |
| Polymer | It is used as a precursor, a chain extender, and a cross-linking agent in producing various polymers. 1. As a precursor, aniline produces polyurethanes, polyamides, and other copolymers, widely used in various applications, including insulation, adhesives, and coatings. 2. As a chain extender, aniline is used to improve the properties of specific polymers. For example, aniline can be used as a chain extender in producing polyurethanes to increase their tensile strength and tear resistance. 3. As a co-curing agent, aniline is combined with other curing agents, such as diaminodiphenylsulfone (DDS) or dicyandiamide (DICY), in the production of epoxy resins to cross-link the polymer chains and form highly durable and heat-resistant materials. |
| Pharmaceutical | Aniline is essential in the pharmaceutical industry as a precursor for synthesizing various pharmaceuticals. One of the most critical uses of aniline in the pharmaceutical industry is in producing analgesics and antipyretics, such as paracetamol (acetaminophen), commonly sold under the brand name Tylenol. Aniline is also used to produce other pharmaceuticals, such as antihistamines, antimalarials, and antibacterials. Its versatility and reactivity make it a functional building block for the synthesis of various drugs in the pharmaceutical industry. |
| Rubber | Aniline has several functions in the rubber industry. It’s an essential building block for synthesizing various rubber chemicals and additives, such as antioxidants, vulcanization accelerators, and stabilizers, added to rubber to improve its performance properties and increase its durability. One such example is aniline, used as a chemical intermediate in synthesizing rubber accelerators, such as N-phenyl-2-naphthylamine (PBN) and N-phenyl-1-naphthylamine (PBNA). Aniline can also be used as an accelerator for certain types of rubbers, such as chloroprene rubber (CR), which is commonly used in the production of wetsuits and diving gears. |
Organic Synthesis
Aniline is used as a reagent in various organic reactions, including preparing isocyanates, nitrobenzene, and chlorobenzene. In these reactions, aniline acts as a nucleophile and reacts with other reactants to produce a wide range of organic compounds.
Preparation of Isocyanates
Aniline can prepare isocyanates by reacting with phosgene or a related compound like triphosgene or diphosgene. Aniline acts as a nucleophile in this reaction and reacts with the phosgene to form an intermediate carbamoyl chloride.
The reaction is:
C₆H₅NH₂ + COCl₂ → C₆H₅NHCOCl + HCl
The carbamoyl chloride intermediate undergoes rearrangement, with the elimination of HCl, to form the isocyanate product.
C₆H₅NHCOCl → C₆H₅NCO + HCl
Isocyanates are essential intermediates in the production of polyurethanes, used in various applications, such as foams, coatings, and adhesives.
Preparation of Methylenedianiline (MDA)
The process typically involves reacting aniline with formaldehyde in the presence of an acid catalyst to form MDA.
C₆H₅NH₂ + HCHO → H₂C(NHPh)₂ + H₂O
Aniline is a starting material in this reaction, which undergoes a condensation reaction with formaldehyde to form MDA. The acid catalyst helps facilitate the reaction by increasing the condensation reaction rate.
Preparation of Chlorobenzene
The conversion of aniline to benzene diazonium chloride and then to chlorobenzene involves two steps:
Step 1: Conversion of aniline to benzene diazonium chloride
The reaction is carried out by treating aniline with nitrous acid (HNO2) in hydrochloric acid (HCl) at a low temperature. The reaction, known as diazotization, results in the formation of a diazonium intermediate.
C₆H₅NH₂ + HNO₂ + HCl → C₆H₅N₂Cl + 2H₂O
Step 2: Conversion of benzene diazonium chloride to chlorobenzene
The diazonium salt is then reacted with cuprous chloride (CuCl) in the presence of hydrochloric acid (HCl) to form chlorobenzene. This reaction is known as the Sandmeyer reaction.
C₆H₅N₂Cl + CuCl + HCl → C₆H₅Cl + CuCl₂ + N₂ + H₂O
Aniline Derivatives
Aniline can be used as a starting material for synthesizing various derivatives through different chemical reactions, including nitration, chlorination, and acylation.
Nitroaniline
The nitration of aniline can synthesize Nitroaniline with a mixture of nitric acid and sulfuric acid. The reaction involves the substitution of a hydrogen atom on the benzene ring with a nitro group (-NO2).
C₆H₅NH₂ + HNO₃/H₂SO₄ → C₆H₅NO₂ + H₂O + H₂SO₄
Use: Nitroaniline finds application as a precursor for synthesizing various dyes and pharmaceuticals.
Chloroaniline
Chloroaniline can be synthesized by chlorinating aniline with chlorine gas or a chlorinating agent such as thionyl chloride or phosphorus pentachloride. The reaction involves the substitution of a hydrogen atom on the benzene ring with a chlorine atom (-Cl).
C₆H₅NH₂ + Cl₂/POCl₃/SOCl₂ → C₆H₅ClNH₂ + HCl
Use: Chloroaniline finds application as an intermediate in producing various pharmaceuticals and agrochemicals.
Anilide
Anilide is an amide derivative of aniline and can be synthesized by the reaction of aniline with a carboxylic acid, such as acetic acid or benzoic acid. The reaction involves the substitution of a hydrogen atom on the nitrogen atom of aniline with an acyl group (-COCH3 or -CO2H).
C₆H₅NH₂ + CH₃CO₂H → C₆H₅NHCOCH₃ + H₂O
Use: Anilide finds application as an intermediate in producing various pharmaceuticals and agrochemicals.
N-Phenylhydroxylamine
N-Phenylhydroxylamine can be synthesized by the reaction of aniline with nitrous acid followed by reduction with sodium sulfite. The reaction involves the conversion of aniline into a diazonium salt, which is then reduced to form N-phenylhydroxylamine.
C₆H₅NH₂ + HNO₂ → C₆H₅N₂⁺ + 2H₂O
C₆H₅N₂⁺ + HSO₃Na → C₆H₅NH₂OH + NaHSO₃
Use: N-Phenylhydroxylamine finds application as an antioxidant and a polymerization inhibitor.
Properties of Aniline
| Chemical Formula | C6H5NH2 |
| Appearance at 25 °C | Colorless to brownish, oily liquid |
| Odor | Characteristic sweet, musty, and fishy odor |
| Molecular Weight (g/mol) | 93.13 |
| Melting Point (°C) | -6.3°C |
| Boiling Point (°C) | 184.13 |
| Density (g/cm³) | 1.02 |
| Refractive Index | 1.5864 |
| Flash Point (°C) | 70-76 |
| Vapor Pressure (mmHg at 77 °F (EPA, 1998)) | 0.67 |
| Solubility | Slightly soluble in water. Soluble in alcohol, benzene, acetone, and most organic solvents. |
| Storage Conditions | Keep in a cool, dry, and well-ventilated area in tightly closed containers made of glass, stainless steel, or other compatible materials to prevent contamination and leakage. Keep away from sources of ignition such as flames and sparks. |
Safety & Regulatory Considerations
| US Information | The EPA outlines a quantitative cancer risk assessment for aniline based on the CIIT study and use of a linearised multistage. Lifetime Permissible daily exposure (PDE) of aniline = 720 µg/day |
| Other Regulatory Information | Aniline is classified by IARC as Group 3, not classifiable as to its carcinogenicity in humans |
Safety & Toxicity of Aniline
According to the harmonized classification and labeling (CLP00) approved by the European Union (EU), aniline is toxic if swallowed, is toxic in contact with skin, is toxic if inhaled, causes damage to organs through prolonged or repeated exposure, is very toxic to aquatic life, causes serious eye damage, is suspected of causing genetic defects, is suspected of causing cancer and may cause an allergic skin reaction.
Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance is very toxic to aquatic life with long lasting effects.
Aniline is a skin and eye irritant and can cause burns upon contact. Always wear appropriate clothing, gloves, and eye protection when handling aniline. Aniline is toxic when inhaled, swallowed, or absorbed through the skin. Avoid breathing in aniline vapors or dust, and avoid skin contact. In case of accidental exposure to aniline, seek medical attention immediately. Ingestion or inhalation of aniline can lead to serious health effects, including liver and kidney damage, blood disorders, and even death. Aniline is flammable and can form explosive mixtures with air. Keep aniline away from ignition sources, and use it only in well-ventilated areas.
Identification Numbers
| Chemical Name | Benzenamine/Phenylamine |
| CAS Number | 62-53-3 |
| EC Number | 200-539-3 |
Acceptable Limits or Maximum Usage
| Category | Usage Level |
| NIOSH Recommended Exposure Limit (REL) | 2 ppm (8 mg/m3) as a TWA (Time-Weighted Average) |
| Occupational Safety and Health Administration (OSHA) | 5 parts per million (ppm) over an 8-hour workday |
Fun Facts About Aniline
- Aniline was first discovered in 1826 by Otto Unverdorben, a German chemist. The name “aniline” comes from the indigo plant (Indigofera anil), from which it was first isolated.
- Aniline was used during World War I as a chemical weapon due to its toxicity and ability to cause skin irritation and blistering.
- Even though aniline has a sweet, pleasant odor when it is pure, as it becomes exposed to air and oxidizes, it quickly turns into a very unpleasant, pungent aroma that can be compared to the smell of rotten fish. This unique and drastic change in odor is due to the formation of aniline’s oxidation products. This property has led to aniline being used as a stink bomb.




