Magnesium stearate is a commonly used ingredient in developing cosmetic products, often added to improve cosmetic formulations’ consistency, texture, and stability.
Magnesium stearate is a white, powdery substance of magnesium and stearic acid. It is a metal salt type often used as a lubricant, binding agent and thickener in cosmetic products. Magnesium stearate is known for its ability to prevent the ingredients in a cosmetic product from sticking together and clumping, resulting in a smooth and consistent texture.
What is Magnesium Stearate?
Magnesium stearate is a compound of magnesium with a mixture of solid organic acids and consists primarily of variable proportions of magnesium stearate and magnesium palmitate. The fatty acids are derived from edible sources. It contains NLT 4.0% and NMT 5.0% of Mg, calculated on a dried basis.
Magnesium stearate is a kind of fatty acid salt type anionic surfactant with a white powder appearance and a slight smell and creamy feeling. It can be soluble in hot aliphatic hydrocarbons, hot arene, and hot grease but insoluble in alcohol and water, decomposing into stearic acid and corresponding magnesium salts in the case of acid. Magnesium stearate has an excellent adhesion property to the skin with excellent lubrication properties. It can be applied to powder products in cosmetics and can improve adhesion and lubrication.
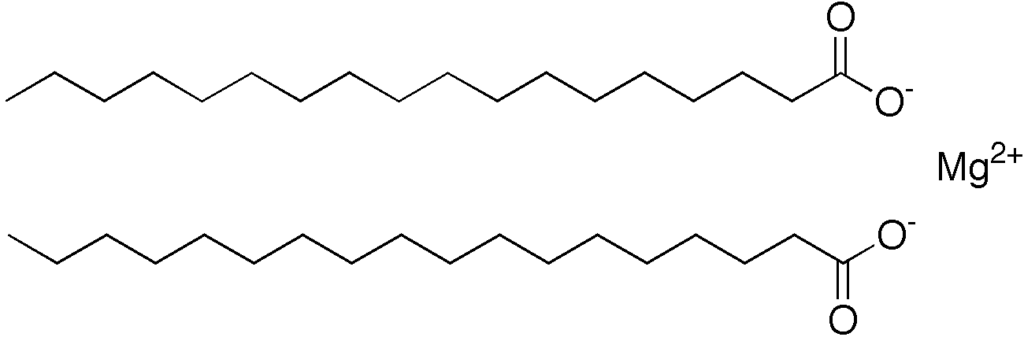
Magnesium stearate chemical structure. Source: Wikipedia
It is a soap consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics.
How is Magnesium Stearate Produced?
Magnesium stearate is prepared either by the interaction of aqueous solutions of magnesium chloride with sodium stearate or by the interaction of magnesium oxide, hydroxide, or carbonate with stearic acid at elevated temperatures.
Magnesium stearate is manufactured from both animal and vegetable oils. Some nutritional supplements specify that the magnesium stearate used is sourced from vegetables. Magnesium stearate is created by the reaction of sodium stearate with magnesium sulfate.
Use of Magnesium Stearate in Personal Care Products
One of the primary benefits of using magnesium stearate in cosmetic products is its ability to improve the stability of the formulation. When used in appropriate quantities, magnesium stearate can help to prevent the separation of ingredients over time, which can prolong the shelf life of a product. This property is essential for products that contain oils, as these ingredients are prone to separation.
Magnesium stearate can also help improve a product’s texture and consistency, such as providing a cream or lotion with a smoother, more velvety feel by reducing the greasy or oily feeling that some products can leave behind. It improves the overall sensory experience of a cosmetic product, making it more enjoyable for consumers.
Applications in Cosmetics
The chemical is used in magnesium stearate-based cosmetic powder surface treatment (a.k.a Magnesium Stearate Pigment Treatment), enabling products with excellent skin adhesion and a moist/creamy texture.
| Function | Applications |
| Texturizing Agent | It helps to give a product a smooth, silky feel and can also reduce the greasy or oily sensation that some products can leave behind. |
| Bulking Agent | It is added to a product to increase its volume or bulk. |
| Anti-Caking Agent | Wide application in color cosmetic applications. Soothing agent in talcum powders and other cosmetics. |
| Lubricant | It helps to give a product a smooth, silky feel and reduces the greasy or oily sensation that some products can leave behind. |
Product Examples
Here are some examples of products that include magnesium stearate:
| Type | Examples |
| Cosmetics | Pressed Powder Makeup, Lipstick, Eye Shadow, Blush, Facial Moisturizer, Body Lotion, Sunscreen |
| Foods | Chewing Gum, Artificial Sweeteners, Dry Mixes, Seasoning Blends |
| Pharmaceuticals | It is primarily used as a lubricant in capsule and tablet manufacture at concentrations between 0.25% and 5.0% w/w. |
Properties of Magnesium Stearate
| Appearance at 25 °C | Light white powder |
|---|---|
| pH (10% Aqueous) | 5-8.2 |
| Molecular Weight (g/ml) | 593.29 |
| Density at 25°C (g/cm3) | 1.026 g/cm3 |
| Solubility | Negligible in water, ether, and alcohol. Slightly soluble in benzene |
| Bulk Density | 0.22 to 0.35 gm/ml |
| Melting Point | 130.00 – 145.00°C |
| Sieve Analysis 200 mesh( passing through) | N.l.t 90% |
| Odor | Faint, characteristic odor |
| Shelf Life | 12-18 months |
| Storage Conditions | Store in well-filled, tightly sealed containers in a cool, dry place. |
Typical Formulations
Magnesium Stearate Formulation Phases
Satin Pearl Eye Shadow – “Sun & Fun” with RICE NS
| PHASE | INCI NAME | TRADE NAME | SUPPLIER | % Wt. |
| A | Talc | Talc Powder | Avantor-VWR | 49.5 |
| DimethylimidazolidinoneRice Starch | Rice NS | Agrana | 4.0 | |
| Mica | Silk Mica | Avantor-VWR | 5.0 | |
| Magnesium Stearate | Magnesium Stearate | Sigma Aldrich | 2.5 | |
| Boron Nitride | Tres BN PUHP 3016 | SAINT-GOBAIN BORON NITRIDE | 3.0 | |
| Mica (and) Titanium Dioxide(and) Tin Oxide | Prestige Soft Orange | Sudarshan | 10.0 | |
| Mica (and) Titanium Dioxide(and) Tin Oxide | Prestige Summer Gold | Sudarshan | 20.0 | |
| B | Ethylhexyl Palmitate | Tegosoft OP | Evonik | 6.0 |
Formulation Procedure
- Mix ingredients of phase A
- Add phase B
- Mix again and stir until uniform
- Press eye shadow at 150 bar for 30 sec
Magnesium Stearate Formulation Considerations
| Things to Avoid | Incompatible with strong acids, alkalis, and iron salts. Avoid mixing with oxidizing solid materials. Cannot be used in products containing aspirin, some vitamins, and most alkaloidal salts. |
Magnesium Stearate Safety & Regulatory Concerns
| FDA Information | GRAS-listed: Magnesium stearate is considered non-toxic and is Generally Recognized As Safe (GRAS) by the U.S. Food and Drug Administration (FDA). Listed on the US TSCA inventory.Included in the FDA Inactive Ingredients Database (oral capsules, powders, and tablets; buccal and vaginal tablets; topical preparations; intravitreal implants and injections).It is approved for use in food and dietary supplements as a lubricant, release agent, emulsifier, binder, thickener, anti-caking, and anti-foaming agent. |
| Other Regulatory Information | In addition to the United States, it is accepted as a safe food additive in Europe, the U.K., and Canada. A specification for magnesium stearate is also included in the Food Chemicals Codex (FCC), a collection of internationally recognized standards for the purity and identity of food ingredients. |
Safety & Toxicity of Magnesium Stearate
- According to the FDA, no evidence suggests that magnesium stearate causes adverse effects when used “at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future.”
- Animal research shows that orally-administered magnesium stearate is non-toxic, far beyond the commonly used amounts. Additionally, as recently as 2015, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) conducted a safety assessment of magnesium stearate and found no concerns regarding its continued use or safety.
- Oral consumption of large quantities may produce a laxative effect or mucosal irritation.
- Included in nonparenteral medicines licensed in the U.K. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Identification Numbers
| Chemical Name | Magnesium Octadecanoate |
| CAS Number | 557-04-0 |
| EC Number | 209-150-3 |
Fun Facts About Magnesium Stearate
- Magnesium stearate is a significant contributor to “bathtub rings”!
- When produced by soap and hard water, magnesium stearate and calcium stearate form a white solid insoluble in water and are collectively known as “soap scum.”
- Despite its name, magnesium stearate is not a true stearate. It is a magnesium salt of stearic acid.
- Magnesium stearate is sometimes called “soapstone” because of its powdery texture and resemblance to soap.






