Avobenzone is a common ingredient in chemical sunscreens thanks to its ability to absorb UVA rays. It has excellent dissolving properties for solid sunscreens, making it especially common in solid product formulations. However, it degrades when exposed to UV rays, limiting its effectiveness. For this reason, it’s combined with other sun protection ingredients to provide longer-lasting protection.
What is Avobenzone?
Avobenzone, also known as butyl methoxydibenzoylmethane, is a derivative of dibenzoyl methane. It has a very high efficacy, even at lower concentrations.Its primary drawback is that its UV protective capability diminishes quickly and significantly due to photodegradation. It must be reapplied every two hours to maintain its effectiveness.
Several approaches have been used to counter avobenzone’s tendency to degrade, including encapsulation, antioxidants, photostabilizers, and quenchers. Combinations of these strategies are typically used.
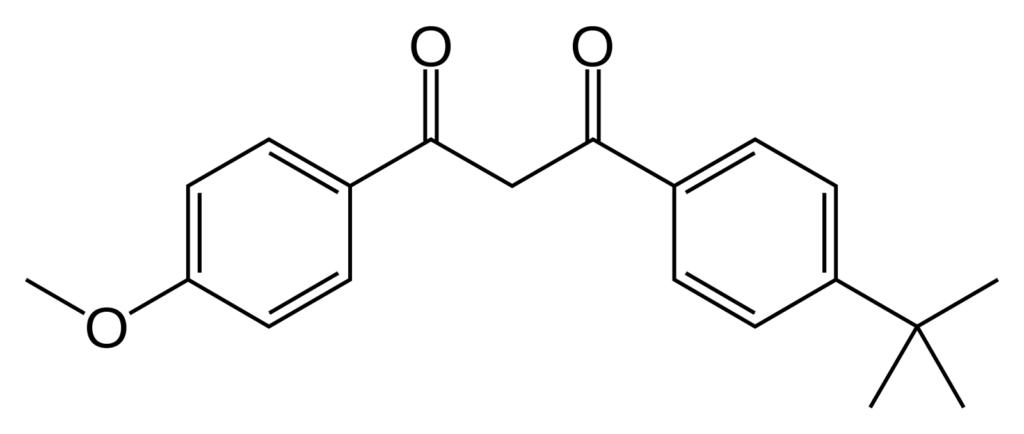
Avobenzone chemical structure. Source: Wikipedia
How is Avobenzone Produced?
Avobenzone is produced by reacting 4-methoxyacetophenone in toluene with 4-tert-butylbenzoic methyl ester in the presence of sodium amide via Claisen condensation (from 4-tert-butylbenzoic acid by esterification with methanol).
Use of Avobenzone in Personal Care Products
Regular exposure to UVA and UVB light can cause considerable skin damage, including DNA damage, photoaging, sunburn, and the development of melanoma and other skin cancers. Sunscreens protect the skin from these damaging impacts. Avobenzone serves the following functions in personal care products.
Applications of Avobenzone in Personal Care Products
Avobenzone functions as a UV protector, UV filter, and UV absorber. Its capabilities are described below.
| Function | Applications |
| UV Protector | When applied to the skin, avobenzone absorbs UV rays. Avobenzone is a UV protector. It is a synthetic compound used to absorb and filter out UVA (ultraviolet A) radiation in sunscreen and other skin care products. Avobenzone is one of the most commonly used UVA filters in sunscreen and other skin care products due to its high efficacy and stability. |
| UV Filter | As a chemical sunscreen, Avobenzone is an Organic UVB filter and an aromatic compound. Its molecular structure is responsible for absorbing UV energy. |
| UV Absorber | Organic filters absorb UV rays, which produce excitation of the sunscreen chemical to a higher energy state. Then, they return to the ground state and convert the absorbed energy into longer, lower energy wavelengths (heat). When applied to the skin, Avobenzone absorbs UV rays and converts it into longer energy wavelengths; thus preventing skin from sun damage. |
Product Examples
Avobenzone is used as a sunscreen in various personal care products and cosmetics. Examples are listed below.
| Type | Examples |
| Sun Protection | Sunscreen Lotions, Creams, Sprays |
| Personal Care | Skin Care Creams, Lotions |
| Cosmetics | Foundations, Lipsticks, Lip Glosses with UV Protection |
Properties of Avobenzone
| Appearance at 25°C | White to pale yellow solid crystalline powder |
| Molecular Formula | C20H22O3 |
| Molecular Weight (g/mol) | 310.39 |
| Density at 20°C (g/cm3) | 1.079 |
| Solubility (at 20°C) | Insoluble in water, slightly soluble in ethanol. Soluble in isopropanol, decyl oleate, capric/caprylic triglyceride, and castor oil |
| UV Specific Extinction(E 1%/1cm) | Max. 1100 at 355±2 nm |
| Melting Point (°C) | 81-86°C |
| Boiling Point (°C) | 463.6±35.0°C |
| Refractive Index (n 20/D, 20°C) | 1.546 |
| Odor | Slight aromatic |
| Shelf Life | 24-36 months |
Recommended Storage and Handling
- Adequate amounts of suitable solvents should be chosen to avoid recrystallization during storage.
- To maximize utilization and high performance, avobenzone should be properly photostabilized.
- Excellent materials for stabilization are octocrylene and 4-methyl benzylidene camphor (or mixtures of these).
- Additional options are polysilicone-15 or benzophenone-3. Use a chelating agent like EDTA.
- Store in well-filled, tightly sealed containers in a cool, dry place.
- Store away from oxidizers: Fire and explosion hazards, protect from light.
Typical Formulations
NuPlastic Sunscreen Lotion
| PHASE | INCI Name | Trade Name | % Wt. |
| A | Water | DI Water | 35.7 |
| Polyurethane 58 | Polyderm PE-PA ED | 16 | |
| Glycerin | Glycerin | 2 | |
| Polysorbate 60 | Tween 60 | 1 | |
| Caprylyl Glycol | Dermol CG | 0.5 | |
| Phenoxyethanol | Phenoxetol | 0.5 | |
| Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer(and) Isohexadecane (and) Polysorbate 80 | Simulgel EG | 0.3 | |
| B | Cetyl Alcohol | Cetyl Alcohol | 2 |
| Stearyl Alcohol | Stearyl Alcohol | 3 | |
| Glyceryl Monostearate | Dermowax GMS | 3 | |
| Avobenzone | Eusolex 9020 | 3 | |
| Octocrylene | Eusolex OCR | 5 | |
| Octisalate | Eusolex OS | 5 | |
| Oxybenzone | Escalol 567 | 6 | |
| Octyldodecyl Neopentanoate | Elefac I-205 | 5 | |
| C12-15 Alkyl Benzoate | Dermol 25B | 2 | |
| C | Isododecane (and) Divinyldimethicone/Dimethicone Crosspolymer | NuLastic Soft ID-6 | 10 |
| Total | 100% | ||
Formulation Procedure
- In a beaker, add Phase A and the thickeners with homogenizer while heating to 70-75° C.
- Mix until smooth and homogenous.
- Add Phase B and heat to 70-75° C in a separate beaker or until everything is melted and dissolved.
- Add Phase B to A under homogenizer to emulsify, then begin cooling to room temperature.
- Below 35° C, add NuLastic Soft and mix until uniform.
Here are the recommended percent weight of Avobenzone in various product formulations:
| Ingredient | % Composition |
|---|---|
| Sunscreen Lotions, Creams, Sprays, After Sun Products, Sunscreen Lotion, Sunscreen Moisturizer, Self-Tanning Products | Up to 3% |
| Skincare Creams, Lotions, Gels | Up to 3% |
| Lipsticks (with UV Protection), Foundation with SPF, Concealer, Highlighters | 1-3% |
Avobenzone Formulation Considerations
- Formaldehyde-donors preservatives should be avoided while formulating with avobenzone as they are incompatible.
- Avobenzone should be added into the oil phase of a cosmetic composition.
- Avobenzone is soluble in some liquid UVB filters and polar oil components. Organic filters, such as a combination of UVA & UVB Filters, are often combined to achieve the desired broad-spectrum protection. For higher SPF protection, avobenzone can be combined with other filters like octocrylene, homosalate, and ethylhexyl methoxycinnamate.
- Irradiation causes the avobenzone molecule to break into radicals, producing chemicals such as arylglyoxals and benzyls that react with other sunscreens. For this reason, avobenzone cannot be used in conjunction with some sunscreens. It should not be used with sunscreens containing Octinoxate as this UV filter can cause a chemical reaction with Avobenzone, reducing its effectiveness.
- Additionally, Avobenzone should not be used with sunscreen that contains mineral oils or petroleum-based ingredients, as these can also reduce the effectiveness of the Avobenzone.
- Chemical Filter-Avobenzone is sensitive to UV radiation. To prevent photodegradation, various photoprotective ingredients should be included in the formulations. Photostabilizers can quench the excited state of the organic filters by accepting the excited state energy, thereby returning the UV-absorbing molecule to its ground state.
- Octyl methoxycinnamate is often used with avobenzone as they both protect against UV radiation but target different parts of the UV spectrum.
Avobenzone is the only long-range chemical UVA filter (340–400 nm) widely available to sunscreen manufacturers in the US. However, the molecule is inherently unstable and loses nearly 50% of its screening capacity after just 1 hour of UV exposure. Furthermore, adding other ingredients, even certain UVB filters, to sunscreens containing avobenzone accelerates the degradation of both compounds. This is why incorporating photostabilizers such as octocrylene is extremely important in formulations.
- UV protection is lost as a result of photodegradation. Avobenzone is still being studied for its photostabilization using various methods depending on the phototoxicity issues, photodegradation mechanism, and compatibility of UV filters.
- Four primary strategies are used to achieve photostabilization:
- Encapsulation: The encapsulation strategy involves using several polymers, such as cyclodextrin. Few compounds have shown effective photostabilization as ROS (reactive oxygen species) trappers, such as UV pearls (encapsulated sunscreens) and microparticles (hollow styrene-acrylate copolymer spheres).
- Implementation of Antioxidants: Adding antioxidants such as Morin; a polymeric form of iron oxide used to absorb and neutralize ultraviolet radiation. Morin is also used to protect the products from oxidation and to enhance the shelf-life of the products; provides synergistic effects through novel drug delivery systems, such as lipospheres.
- Utilization of Photostabilizers: Octocrylene can be used as a photostabilizer.
- Quenchers: More exploration is required to develop suitable quenchers to induce proper photostabilization of avobenzone.
Avobenzone Regulatory Considerations
Homosalate is authorized worldwide. However, avobenzone can harm coral reefs.
| FDA Information | Avobenzone is approved as an active ingredient in over-the-counter (OTC) sunscreen drug products. The authorized range for avobenzone in cosmetics is up to 3% when combined with other sunscreens per monograph. It is approved for suncare preparations at a maximum concentration of 3%. |
| EU Information | The recommended dosage of avobenzone given by the European Union’s Cosmetic Directive is up to 5%. |
| Japan Information | Authorized range is up to 10% (leave-on application). |
| Asia Information | Recommended dosage is up to 5% in leave-on applications. |
| Canada Information | Recommended dosage is up to 5%. |
| South Africa Information | Authorized dosage is up to 5%. |
Identification Numbers
| INCI Name | Butyl Methoxydibenzoylmethane |
| CAS Number | 70356-09-1 |
| EC Number | 274-581-6 |
| EINECS/ELINCS No | 274-581-6 |
| COSING REF No | 31713 |
Fun Facts About Avobenzone
- Avobenzone was approved by the FDA in 1996.
- Because avobenzone stands alone as a chemical sunscreen that protects against UVB and has excellent effective properties, research is being prioritized to discover new ways to prevent the degradation process.
Additional Sources & Resources
- ScienceDirect – Zofenopril Calcium
- HKTDC Research – Pharmaceutical Industry in China
- ScienceDirect – Solid-state NMR characterization of drug substances and formulations
- MDPI – Novel Co-Amorphous Drug Formulations
- ChemicalBook – Zofenopril Calcium
- ChemicalBook – Zofenopril Calcium Properties
- FDA – List of Bulk Drug Substances for Which There is a Clinical Investigation
- INTRODUCTION TO COSMETIC FORMULATION AND TECHNOLOGY by GABRIELLA BAKI, Ph.D. AND KENNETH S. ALEXANDER, Ph.D. The University of Toledo, College of Pharmacy and Pharmaceutical Sciences- Chapter 3- Skin Care Products, Section 5- Sun Care Products; Page 275









