Dichloromethane’s unique physical and chemical properties make it an essential tool in various industrial applications, from the manufacturing of pharmaceuticals to the removal of paint. Its physicochemical properties, high volatility, low boiling point, and ability to dissolve a wide range of organic compounds lend it exceptional versatility.
This article explores the diverse industrial applications, properties, and regulatory considerations for product developers considering dichloromethane in their formulations.
What is Dichloromethane?
Dichloromethane (DCM), methylene chloride, is a colorless, volatile organic compound (CH₂Cl₂). With a sweet and pleasant aroma, it is typically utilized as a solvent in various industrial and laboratory operations. Denser than air and boasting a boiling point of 39.8°C, dichloromethane is an effective solvent for extracting compounds sensitive to elevated temperatures. Additionally, it is frequently employed as a paint stripper, degreaser, and in producing foam plastics and resins.
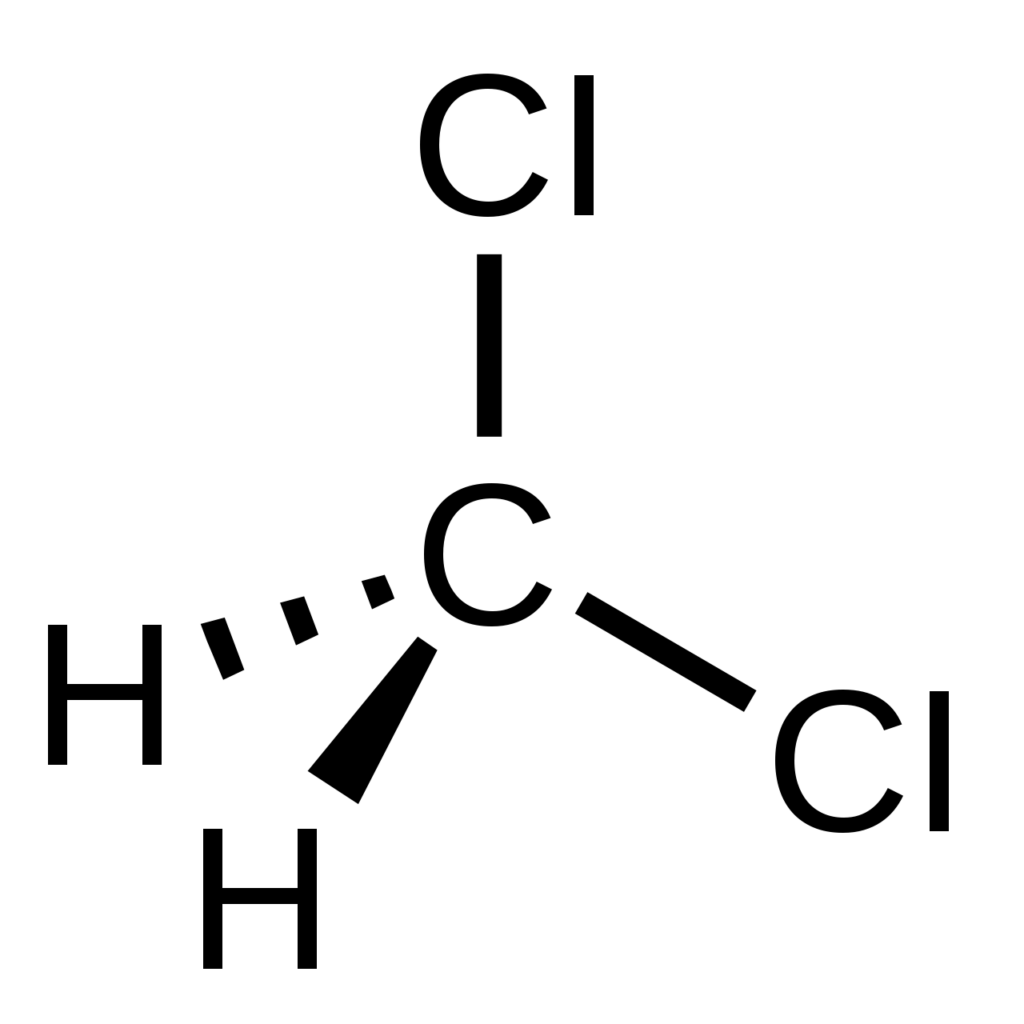
Source: Wikipedia
How is Dichloromethane Produced?
Dichloromethane doesn’t naturally appear in significant quantities within the environment, being primarily a synthetic chemical created via industrial processes. Nevertheless, minimal amounts of dichloromethane can be detected in the atmosphere due to natural sources like volcanic eruptions, forest fires, and oceanic emissions. Dichloromethane has also been found in certain marine organisms and types of fruits, such as guavas and mangoes, presumably resulting from environmental contamination. However, these concentrations are generally relatively low. Altogether, dichloromethane is an industrial chemical infrequently found in nature.
Chlorination of Methane:
The chlorination of methane is a chemical reaction wherein methane interacts with chlorine gas under the influence of UV light or heat to yield chloromethane. This reaction advances through several radical intermediates. Initially, chlorine is homolytically cleaved to create chlorine radicals. These radicals react with methane to produce a methyl radical and hydrogen chloride. The methyl radical can then interact with a chlorine molecule to generate dichloromethane.
Step 1: Cl₂ → 2 Cl• (homolytic cleavage)
Step 2: Cl• + CH₄ → CH₃• + HCl (initiation)
Step 3: CH₃• + Cl₂ → CH₃Cl + Cl• (propagation)
Overall reaction: CH₄ + Cl₂ → CH₃Cl + HCl (overall)
The chloromethane molecule can undergo further chlorination to produce dichloromethane.
CH₃Cl + Cl₂ → CH₂Cl₂ + HCl
Most dichloromethane is manufactured in large-scale industrial facilities, frequently a byproduct of other chemical processes such as chlorinated solvents, plastics, and synthetic fibers.
Dehydrochlorination of Chloroform
The conversion of chloroform (CHCl₃) to dichloromethane (CH₂Cl₂) using hydrochloric acid (HCl) and zinc (Zn) in ethanol.
CHCl₃ + Zn + 2 HCl → CH₂Cl₂ + ZnCl₂ + HCl
In this reaction, zinc metal (Zn) acts as a reducing agent, and hydrochloric acid (HCl) serves as a dual source of hydrogen atoms and a catalyst. Ethanol is used as the solvent for the reaction. The reaction unfolds through a series of reduction stages, wherein chloroform is reduced to dichloromethane by the hydrogen atoms from HCl, and zinc is oxidized to zinc chloride (ZnCl₂).
Uses and Applications of Dichloromethane
Dichloromethane is employed extensively with various organic compounds, including fats, oils, waxes, resins, and numerous polymers. Below are some common uses and applications:
Chemical Manufacturing: Dichloromethane is a popular solvent in manufacturing polycarbonate resins and polyurethane foams. Its usage in polycarbonate resin production allows for precise control over reaction conditions like temperature, pressure, and reactant concentrations, yielding high-quality, consistent products. These resins find uses in diverse applications, including automotive parts, electrical components, and household items such as water bottles and food containers.
For polyurethane foam production, dichloromethane is employed as a blowing agent. This category of foams is used across various applications, including insulation, packaging, and cushioning materials. When dichloromethane is added to the polyurethane foam mixture, it vaporizes, creating gas bubbles that expand the foam to produce a lightweight, high-performance material.
Adhesives: Dichloromethane is used in adhesives as a solvent to dissolve the resin or polymer employed to make the adhesive. This solvent creates a solution that can be applied to surfaces requiring bonding. A key advantage of using dichloromethane in adhesives is its rapid drying time. Its fast evaporation enables quick bonding of characters, which is especially useful in applications demanding a swift cure time, such as in automotive manufacturing or construction, and notably in the production of contact adhesives or solvent cement.
Paint Stripping: Owing to its ability to dissolve an extensive range of organic and inorganic compounds, dichloromethane is a frequent ingredient in paint removers and strippers. However, its usage in paint strippers has been a topic of concern due to potential health risks.
Aerosol Propellant: Dichloromethane is used as an aerosol propellant in several aerosol products, like spray paints, cleaners, and lubricants. It rapidly vaporizes when the aerosol can’s valve is opened, generating pressure that forces the product out of the can.
Pharmaceuticals: The pharmaceutical industry extensively uses dichloromethane as a solvent for diverse applications. It is a versatile reaction solvent capable of dissolving a broad spectrum of organic compounds. Its low boiling point makes it easy to remove from the final product.
Typical applications of dichloromethane in the pharmaceutical industry include extracting active pharmaceutical ingredients (APIs) from natural sources and cleaning equipment, containers, and surfaces. Its excellent solvency and cleaning properties also make it widely used in the metal cleaning industry.
Food Industry: Traditionally, dichloromethane has been used in the food industry for decaffeinating coffee and tea. It’s a strong solvent that can efficiently remove caffeine from these beverages. However, its use is becoming less common due to health and environmental concerns.
Laboratory Uses: Dichloromethane is commonly used as a solvent for various chemical reactions in the laboratory. It is handy for extracting complex compounds from biological samples. It is also a common solvent for chromatography techniques such as thin-layer (TLC) and high-performance liquid chromatography (HPLC).
Properties of Dichloromethane
| Molecular formula | CH2Cl2 |
| Appearance | Colorless transparent liquid |
| Molecular weight ( g/mol) | 84.93 |
| Boiling point ( °C) | 39.6 |
| Melting point (°C) | -96.7 |
| Density (g/cm3) | 1.33 |
| Vapor pressure (mmHg at 24 °C) | 400 |
| Dielectric constant | 8.93 |
| Refractive index | 1.424 |
| Solubility | Moderate water solubility. Soluble in most organic solvents such as ethanol, ether, phenols, aldehydes and ketones. |
| Storage conditions | Store in a cool, dry, and well-ventilated area. Avoid incompatible materials, such as strong acids, bases, or oxidizing agents, as this can cause a chemical reaction that may lead to fire or explosion. Avoid using containers made of aluminum or brass, as these can react with dichloromethane and cause contamination or degradation. |
Dichloromethane Derivatives
Chloroform: This compound is synthesized by reacting dichloromethane with sodium hydroxide. The reaction occurs through a dehydrohalogenation process, where the hydroxide ion targets the hydrogen atom attached to one of the dichloromethane’s chloride groups. This interaction results in the formation of a chloride ion and a carbanion intermediate. This unstable intermediate spontaneously breaks down to form chloroform and a hydroxide ion.
CH₂Cl₂ + 2NaOH → CHCl₃ + Na₂CO₃ + H₂O
Usage: Chloroform, a colorless, sweet-smelling liquid, is frequently used as a solvent, anesthetic, and refrigerant.
Methyl Chloroformate: This derivative is created by reacting dichloromethane with methanol and phosgene. In this reaction, phosgene interacts with methanol to produce methyl chloroformate and hydrogen chloride.
CH₂Cl₂ + CH₃OH + COCl₂ → CH₃OCOCl + 2HCl
Usage: Methyl chloroformate, an organic compound, is used in synthesizing pharmaceuticals, agrochemicals, and perfumes.
Safety & Regulatory Considerations
| OSHA | The legally permissible exposure limit (PEL) for airborne dichloromethane is 25 parts per million (ppm), averaged over an 8-hour work shift. This limit should not exceed 125 ppm during any 15-minute work period. |
| NIOSH | This agency recommends limiting exposure to occupational carcinogens to the lowest feasible concentration. |
| ACGIH | The threshold limit value (TLV) set by this entity is 50 ppm, averaged over an 8-hour work shift. |
| IDLH | The revised immediate-danger-to-life-and-health (IDLH) value for methylene chloride, another name for dichloromethane, is 2,300 ppm, as it is based on acute inhalation toxicity data in humans [Sax 1975]. |
Safety & Toxicity of Dichloromethane
Dichloromethane irritates the skin, eyes, and respiratory tract. Exposure to high levels of dichloromethane vapor can cause dizziness, headaches, nausea, and in severe cases, unconsciousness, and even death. Long-term exposure to dichloromethane can cause liver and kidney damage and an increased risk of certain types of cancer. Prolonged or repeated skin contact with dichloromethane can cause dryness, cracking, and dermatitis.
Toxicity Information
Toxicity from exposure to DCM can be brought on by either the substance’s direct effects or via its metabolite, carbon monoxide. Dichloromethane is classified as a Category 2 carcinogen by the International Agency for Research on Cancer (IARC) due to its potential to cause human cancer. It is also considered a developmental and reproductive toxin, and exposure to dichloromethane during pregnancy may cause harm to the fetus.
Acute toxicity studies in animals have shown that high doses of dichloromethane can cause damage to the liver, kidneys, and lungs, as well as central nervous system effects such as sedation, ataxia, and convulsions. EPA has classified dichloromethane as a probable human carcinogen.
Identification Numbers
| Chemical Name | Dichloromethane |
| CAS Number | 75-09-2 |
| EC Number | 200-838-9 |
Fun Facts About Dichloromethane
- Dichloromethane was discovered by the French chemist Jean-Baptiste Dumas in 1839 while he was synthesizing chloroform. Dumas had been trying to synthesize chloroform from chlorine and ethanol. Still, he noticed that another compound was forming in the process, which turned out to be dichloromethane.
- During the Second World War, it was necessary as an industrial chemical & afterward, global production of dichloromethane continued to rise from 93000 tonnes in 1960 to an estimated 570000 tonnes in 1980.
- When burnt, the flame of dichloromethane is nearly invisible. This unique property can make it particularly hazardous when improperly handled.
- Dichloromethane is about 2.5 times denser than air, so it can concentrate in low-lying areas if released.
- At room temperature, dichloromethane is a volatile liquid, meaning it evaporates quickly into the air.